Adnan Omran Alsawehli1,2, Mohd Sahaid Kalil2, Salem. M. Alburki1 ,Jmal Khlifa Alfeluo1,2
1Chemical Engineering Department, Faculty of Engineering, El-Mergib University, Al-Khums, Libya
2School of Chemical and Process Engineering, National University of Malaysia, Selangor, Malaysia
Adnan0102a@gmail.com , smalburki@elmergib.edu.ly,
Corresponding Author: Adnan0102a@gmail.com
HNSJ, 2021, 2(11); https://doi.org/10.53796/hnsj21110
Published at 01/11/2021 Accepted at 05/10/2021
Abstract
Bacterial cellulose (BC) is a unique biopolymer with a high crystallinity, strength and high purity which make it gaining extensive interesting and has a commercial applications in a variety of industries. This study aims at investigation the potential of Acetobacter xylinum to produce BC from the coconut water and the effects of initial pH, initial substrate (glucose) concentration and incubation temperature were also studied the ability of coconut water without additional substrate. All the fermentation processes were carried out in a static culture. Besides that, the physical properties of the BC produced at the optimum conditions from coconut medium were evaluated. Also, the structure and morphology of the BC produced were evaluated using Scanning Electron Microscope (SEM). Results showed that the initial pH, initial substrate concentration and incubation temperature to optimally produce biocellulose were 4.0, 20.0 gL-1 and 30oC respectively and the maximum amount of BC produced was 27.096 (gL-1). The highest amount of biocellulose produced by using coconut water without additional substrate was 13.246 (gL-1). The thickness of biocellulose pellicles varied from 0.1215 – 0.760 mm in the dry state and from 1.509 – 8.351 mm in wet state. The diameter of the pellicles varied from 80-82 mm, this was dependent on the surface of the medium. Water Holding Capacity (WHC) varied between 11% – 74% and moisture content was in the range of 92–98%.
Key Words: Acetobacter xylinum, Bacterial cellulose, coconut water.
1- Introduction
The production of processed foods and the consequent demand for functional ingredients has expanded dramatically in recent years. Cellulose and cellulose derivatives are versatile multi-functional food ingredients. Wood pulp and cotton linters are the raw materials for the production of microcrystalline cellulose and cellulose derivatives. The cellulose produced by Acetobacter xylinum is an alternative source. Unlike the cellulose from wood pulp, bacterial cellulose is devoid of other contaminating polysaccharides, and its isolation and purification are relatively simple, not requiring energy- or chemical-intensive processes, Further, environmental problems due to by-products of wood pulping given an added impetus to study unexplored sources of cellulose (Embuscado et al., 1994). Bacterial cellulose produced by A. xylinum has properties that make it suitable for use in different fields. When compared with plant cellulose, its most striking features are its greater water-holding capacity, higher crystallinity, tensile strength, and a good biocompatibility. These features are a result of the nanostructurated network formed by its microfibrils (Goelzer et al 2008).
Acetobacter bacteria have been commonly found in symbiotic relationships with many different plants such as sugarcane and coffee plants and consequently isolated. Acetobacter xylinum is a gram-negative, aerobic bacterium that has long served as a model organism for the study of bacterial cellulose synthesis; primarily because of the large quantities it produces. A single A. xylinum cell is capable of polymerizing 200 000 glucose molecules per second into β-1,4-glucan chains which are then excreted into the surrounding medium forming ribbon-like bundles of microfibrils2. The produces crystalline fibers resemble in width and structure average fibrils form many plants and algae (Ross, P at al 1991)and . (Ślusarska at al 2008)
2-MATERIALS & METHODS
2-1 Microorganism and culture media
Acetobacter xylinum obtained from the laboratory of Biotechnology, Chemical Engineering Department (University Kebangsaan Malaysia). The culture was maintained on a coconut water agar and stored in the refrigerator (4oC). The culture was transferred to coconut water agar every 14 days. It was incubated at room temperature (about 30oC) for 3 days prior to keeping in the refrigerator (4oC) (Phunsri et al 2003). The coconut water glucose medium (CWG) consisted of 20.0 g glucose, 5.0 g (NH3)2 SO4, and 5.0 g glacial acetic acid dissolved in 1.0 L of coconut water. Coconut water was obtained from coconut purchased locally from Malaysia (Kagang). The initial pH was adjusted to 4, 5 and 6 with 0.1 M HCl or sterilized 1 M NaOH. The medium transferred to conical flasks 250 mL were autoclaved for 20 min at 121 oC and 15 psi, then the medium cooled to a required temperature for the fermentation process or stored at 4 oC in the refrigerator. Table 1 presents chemicals and composition of fermentation media used in this study.
Table 1. chemicals and composition of fermentation media used in this study
| Material | Manufacturer | Composition of Solid Medium Agar (Isawano et al 2002) | Composition of CWG Medium (verschuren. et al 2000) | ||
| Glucose, C6H12O6 | Chempur, Germany | Glucose | 20.0 g/L | Glucose | 20.0 g/L |
| Ammonium sulfate, (NH3)2 SO4 | R & M Marketing, Essex, United Kingdom. | Agar | 20.0 g/L | (NH3)2 SO4 | 5.00 g/L |
| Acetic acid, CH3COOH | BDH Chemical Ltd Poole England | (NH3)2 SO4 | 5.00 g/L | Acetic acid | 5.00 g/L |
| Sodium hydroxide, NaOH | Progressive Co., Malaysia | Acetic acid | 5.00 g/L | Coconut water | 1.000 L |
| Bacteriological agar | Becton, Dickinson and Company, USA | Coconut water | 1.000 L | Glucose | 20.0 g/L |
| Sodium Bi-phosphate, Na2HPO4 | Weifang Huabo Chemical Co., Ltd, China | Glucose | 20.0 g/L | ||
| Peptone | Oxoid, England | ||||
| Citric acid, C6H8O7 | John Kollin Corporation, USA | ||||
| Yeast extract | Becton, Dickinson and Company, USA | ||||
| Coconut water | Purchased locally from Malaysia (Kagang) | ||||
2-2 Bacterial Growth Pattern and Fermentation Process
The Liquid media can be solidified with agar. Prepare solid media (Agar) by dissolving the agar in the liquid medium, the composition of the medium . After dissolved the agar in the medium, heated at a temperature from 70-90 oC until the homogenous, after that the medium autoclaved at 121oC from 15-20 min and 15 psi to sterilize the medium, then Cool the agar to about 50°C, at this temperature the medium will stay in liquid indefinitely, but it will rapidly solidify if it temperature falls much below 45°C. Then Pour the medium into sterile disposable Petri dishes (plates) and allow solidifies. Transferring the agar to the plates must be achieved under the most sterile conditions we’re able to achieve. The next step was achieved when the agar is completely frozen, after that transfer number of bacteria by using inoculating Loop to the surface of agar, the loop sterilized by holding it in a Bunsen burner flame until it is red hot. Cool the loop by touching it to a sterile portion of the surface of an agar plate until it stops sizzling. After inoculating the plates are closed by wrapping the parafilem around the plate. Finally, the plates incubated at 30 oC for 3 days, now the solid medium ready for preparing the stock culture or kept in the cool room at 4 oC. The A. xylinum produced is transferred to the liquid medium in 1:10, where each of 10 ml A. xylinum was added to a 90 ml of sterile coconut medium. Then, the culture medium incubated at 30 oC for 3 days. After that the bacteria is transferred into a Petri dish of coconut solid medium under the most sterile conditions we’re able to achieve as outlined in the preparation of solid medium. The stock culture prepared by transfer the colonies from the Petri dishes to sterile liquid coconut medium and incubated at 30 oC for 3 days. Finally, the stock culture is ready for the fermentation process.
3- ANALYSIS
The analysis procedure of bacterial cellulose production was summarized as shown in Figure 3.4. The analysis including: dry weight of bacterial cellulose, final pH value and final substrate concentration (glucose). Moreover the bacterial cellulose produced was characterized assuming the following criteria: the yield of the biosynthesis process in variable conditions and the characteristics of bacterial cellulose, including a measure the water holding capacity, moisture content, diameter and thickness of pellicles .
3-1 Determination of pH Values and Glucose Concentration
Determining the final pH value was determined using a pH meter (EUTECH, Singapore). The pH meter calibrated with a standard buffer solution before being used to measure the pH value of the sample.Glucose concentration was determined using Biochemistry Analyzer (YSI Model 2700 SELECT, USA). Prior to the analysis, 1 ml from the medium was diluted ten times with distilled water then the solution transfer to a microtube 1.5 mL (Eppendroff tube). This tube was centrifuged using centrifuge machine (3000 rpm for 5 min). After that the sample was detected by Biochemistry Analyzer as shown in figure 3.3.
Glucose concentration = reading 10 = ( ) g/L, where 10 is the dilution factor.

Figure 3.3: Biochemistry Analyzer for Measure Glucose Concentration
3-2 Determination the Amount of Bacterial Cellulose Produced
During the breeding of Acetobacter xylinum bacteria in stationary conditions, bacterial cellulose was produced and synthesized in the form of a pellicle on the surface of the nutrient medium solution in flasks. The bacterial cellulose removed after the period of formation and treated (Shezad et al 2010).
3-3 Treatment of Bacterial Cellulose
The obtained (bacterial cellulose pellicle) from the culture flask was purified by washing repeatedly with large quantities of distilled water. Then the pellicle of biocellulose was treated by immersion in a NaOH (a concentration of approx. 1%, for 20 min., temp = 100 °C) in order to remove bacterial cells, residual medium from the inner layers of the bacterial cellulose and other impurities (Phunsri et al 2003). Next, bacterial cellulose once again washed with distilled water, and kept in distilled water in a refrigerator at 4 oC for the next steps.
3-4 Determination Dry Weight of Bacterial Cellulose
The amount of bacterial cellulose in the pellicles was measured by boiling the harvested pellicle in 0.1N NaOH for 20 minutes as outlined. Then the pellicles were soaked in water for at least two hours and placed for drying in oven at a temperature of 70oC until the weight did not change. The weight of dried pellicles was expressed as bacterial cellulose weight.
3-5 Measurement of Water Holding Capacity (WHC) of Bacterial Cellulose
The water holding capacity of the samples was measured using the shake method (Schrecker, S. T et al 2005). Pellicles of bacterial cellulose were removed from the flasks by using tweezers. The samples were shaken twice quickly and then weighed. Then, bacterial cellulose samples were dried for 12h at 70◦C in order to completely remove water from them. Finally, they were transferred quickly to the balance for weighing (Shezad et al 2010). The water holding capacity was calculated using the following formula:
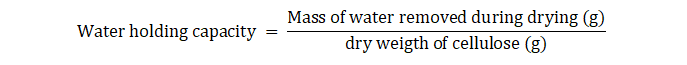
3-6 Measurement of Wet Weight and Moisture Content
The bacterial cellulose obtained was allowed to drip for 30 s and the wet weight was determined (Watanabe, K et al 1995). Moisture content is the amount of the water in the bacterial cellulose pellicle. The moisture content (%w/w) of bacterial cellulose was determined based on the weight loss of the pellicle when dried in an oven at a temperature of 70oC until the weight did not change.
The moisture content is calculated by using this formula:
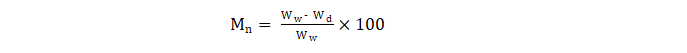 Where:
Where:
Mn = moisture content (%) of material n
Ww = wet weight of the sample
Wd = weight of the sample after drying.
3-7 Determination of Thickness and Diameter of the Bacterial Cellulose Pellicl
Thickness of the bacterial cellulose pellicles was determined using a Sylvac Digital Micrometer (S-Mike PRO, Germany) as shown in figure 3.4. Where the sample (pellicle) of bacterial cellulose placed between two plates and put between the measuring surfaces in the hard metal of the micrometer after that the pellicle was compressed twice in a reciprocating motion that imitated the action of the jaw, the Adjustable measuring force 5N / 10N. The reading in the micrometer indicates the thickness (mm) of the pellicle, where the measuring ranges of the Sylvac micrometer from 0-102 mm. The Pellicles diameter was determined by using listed ruler to measure the diameter.
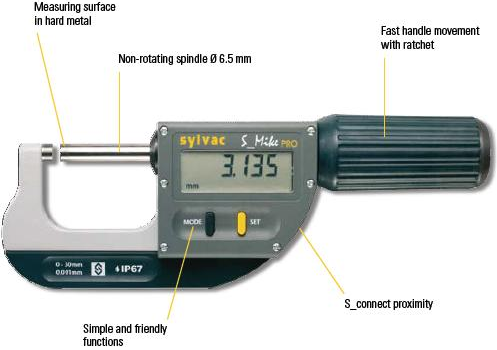
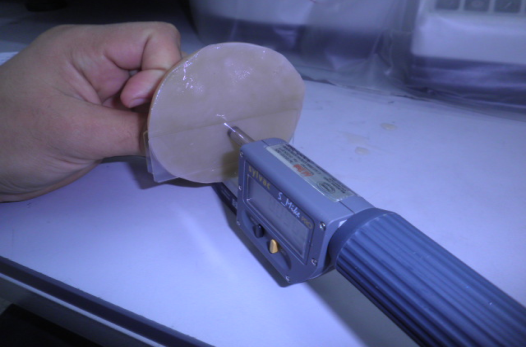
Figure 3.4 Sylvac Digital Micrometers for Measure the Thickness (mm) of Bacterial Cellulose Pellicles.
3-8 Bacterial Cellulose Scanning Electron Microscopy (SEM) Analysis
Scanning electron microscopy (SEM) images of bacterial cellulose was taken using SEM (LEO 1450VP, LEO Co. LTD) to study the structure and the morphology of the bacterial cellulose. The morphology of the samples was investigated for the bacterial cellulose pellicles that produced at the optimum conditions, where 8 pellicles were collected. The sample was taken each day for 8 days of the incubation. Prior to SEM analysis, the samples treated and washed twice with distilled water to remove the impurities and the residual substrate. The samples were prepared for the SEM in two cases. In the first case, after the bacterial cellulose dried by oven at 70oC and in the second case, the samples were rinsed in distilled water after treated and dehydrated by freeze-dryer (Alpha 1-4 LSC Freeze dryer, Germany) at −40 °C and 0.050 mbar. All the samples that were prepared for the SEM were sputter coated with gold and the cell morphology was examined using SEM.characterization analysis including the scanning electron microscope (SEM).
4- Results and Discussion
4-1 Biocellulose production from coconut medium at different glucose concentration
Three different glucose concentrations were used 15, 20 and 25 g/L. The media was prepared as outlined and transferred into conical flasks 250 mL, where 8 conical flasks for each concentration was prepared and incubated at 30 oC and pH adjusted at 4.75. Each 24 h the sample was taken and the period of fermentation was 8 days. Biocellulose and biomass produced on the surface of the flask it was removed and treated to obtain pure biocellulose. Figure 4.1 it shown the growth rate of biocellulose at different glucose concentrations in the coconut medium. The pH was initially adjusted 4.75, where the period of fermentation of coconut medium for biocellulose production was 8 days, where it was proved that the greatest increase in the weight of bacterial cellulose takes place after 7 – 8 days of breeding Acetobacter xylinum (Ślusarska et al 2008). The amount of biocellulose produced from the fermentation of various sugar concentrations was increased with the time. The biocellulose pellicles were collected for each day of fermentation.
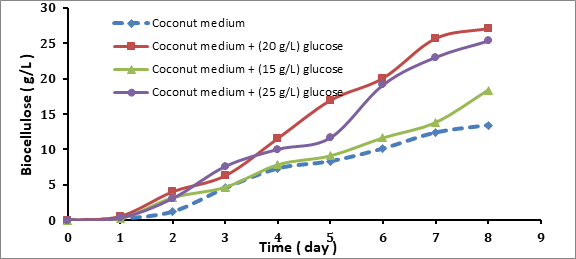
Figure 4.1 Biocellulose Productions (g/L) at Different Glucose Concentrations Medium
The biocellulose productions were 18.389, 27.096 and 25.319 g/L biomass when the glucose concentration was 15, 20 and 25 g/L, respectively. Besides that, in the coconut medium without adding substrate (glucose), the biocellulose production was 13.425 g/L. It was observed that the biomass produced effected by the concentration of the sugar (glucose). The results showed that 20 g/L of glucose was the optimum concentration and gained the highest production of biocellulose. Also, it was observed that the coconut medium without adding glucose has the ability to produce biocellulose as 13.425 g/L dry weight after 8 days fermentation.
The pH reading of the end of each day from the fermentation for different glucose concentration medium is shown in Figure 4.2. It was observed that there is a decreasing in pH value along with the incubation time regardless of the glucose concentration. The decreasing pH values in the initial 15 g/L glucose concentration is less than in 20 g/L and 25 g/L glucose medium. While the decreeing in pH at initial 20, 25 g/L of glucose is almost the same. The pH decreases during fermentative production because of the accumulation of gluconic, acetic or lactic acids in the culture broth, the conversion of glucose to gluconic acid led to a significant drop in pH of the culture broth (Phunsri et al 2003). The bacteria consumed the substrate (glucose) for built and produce a nanostructurated network (biocellulose) on the surface of the broth, where the bacteria convert the glucose to gluconic acid which that leads to decrease the concentration of glucose gradually as shown in figure 4.3. In a previous study by Angkana, it was reported that the reducing sugar could be utilized for the production of gluconic acid, which is the product of glucose oxidation by Acetobacter (Phunsri et al 2003). Also it was reported that by Young kook yang (yang et al 1995), the A. xylinum oxidized a portion of glucose to gluconic acid, with the accumulated gluconic acid lowering the pH of the culture medium and inhibiting cellulose production.
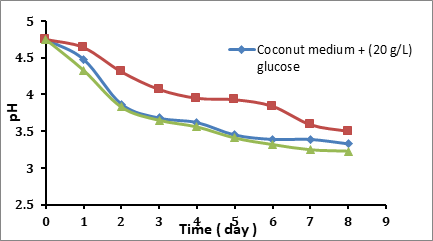
Figure 4.2 Final pH values from Different Glucose Concentration in the Coconut Medium
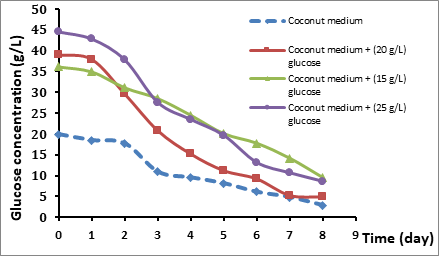
Figure 4.3 Glucose Concentrations during the course of Fermentation of Coconut Medium
4-2 Influence of temperature on the biocellulose production
Four different temperatures were used, namely 25, 30, 35, and 40 oC. The optimum growth parameters were used as pH 4.75 and 20 g/L glucose concentration. The experiments carried out using conical flasks 250 mL with working volume of 100 mL and after the inoculation (fresh inoculum of A. xylinum 10%) the flasks were incubated for 8 days at different incubation temperatures.
From Figure 4.4, it was observe that for the breeding conducted at 30, 25, 35 and 40°C; it clearly appears that the optimum temperature is 30 oC in which the maximum amount of biocellulose was produced as 26.036 g/L. Also, it was observed that the biocellulose yield at 25 °C after 8 days was 20.902 g/L. It was approximately higher than the biosynthesis yield at 35 and 40 °C, where the yield was 18.74, 2.007 g/L, respectively.
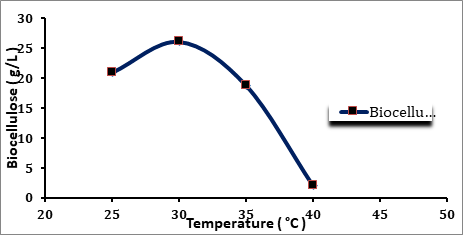
Figure 4.4 Effects of the Temperature on the Biocellulose Production
Temperature is a crucial parameter that affects both growth and cellulose production. These were in accordance with conditions determined in the preliminary studies. In the majority of experiments, the maximal cellulose production was observed between 28 and 30 °C as reported by Prashant (Prashant R et al 2009). Also it was proved that by Barbara (Ślusarska et al 2008), the greatest increase in the weight of bacterial cellulose takes place after 7 – 8 days of breeding Acetobacter xylinum at a temperature of 30 °C.
4-3 Effect of initial pH on the production of biocellulose
Three different initial pH were used, namely 4, 5, and 6. These experiments were conducted at the optimum growth parameters, 30 oC and 20 g/L glucose concentration with change in initial pH value only. The experiments were carried out for 8 days in an incubator without shaking (statically).
The effect of pH on the production biocellulose was presented in figure 4.5. The amounts of biocellulose were produced from the fermentation of various initial pH were collected. At the initial 4.0 pH, the amount of biocellulose was produced as 26.139 g/L, while pH 5.0 was resulted in 24.987 g/L and pH 6.0 was resulted in 16.703 g/L of biocellulose. In these experiments it was observed that the change in the initial pH of the fermentation medium had an effect on the growth of A. xylinum. The optimum pH for the production of biocellulose was found to be 4.0. As reported by Peter, the pH for optimal cellulose production and bacterial growth is between 4.0 and 5.0. Our results are in agreement with that reported previously by Peter (Peter et al 2000). Furthermore, as reported by Masaoka (Masaoka et al 1993), the optimum pH of the Acetobacter xylinum was found to be 4.75 which are in the range of 4-6.
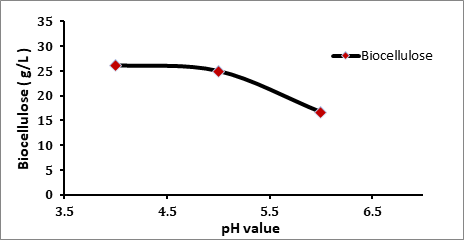
Figure 4.5 The Effect of Initial pH Value on the Biocellulose Production
4-4 Effects of inoculum on the growth rate of acetobacter xylinum and the produceion of biocellulose using coconut water medium
From Figure 4.6 it was observed that the change inoculum has a significant effect on the growth rate of the bacterial cellulose especially in the first 24 h. From the Figure it can see that when the inoculum was grown on HS medium and used to inoculate the coconut medium, no product was appeared after 24 h. After 2 days the bacteria started to produce biocellulose which was 2.643 g/L, while after 8 days from the fermentation, the maximum product was 17.050 g/L. On the other hand when the coconut medium was used to prepare the inoculum and as a production medium, the product was appeared after 24 h as 0.567 g/L and after 2 days as 4.037 g/L. The maximum production was 27.096 g/L after 8 days. When HS was used to develop the inoculum there is no apparent growth in the first 24 h because the bacteria adapt themselves to new growth conditions. It is the period where the individual bacteria are maturing and not yet able to divide. This period called lag phase. This period is reduced when coconut inoculum used and the inoculum has the same composition of the medium then the bacteria adapt directly with the new environment to produce Biocellulose. The previous studies have been proved that there is no apparent net growth observed during the lag phase.
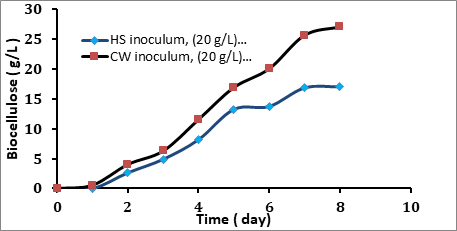
Figure 4.6 Effect Inoculum on the Coconut Water Medium Fermentation
4-5 Characterization of the biocellulose produced
This experiment conducted to characterize the pellicle of biocellulose produced in order to identify the characteristics of the pellicle where the experiment carried out under the optimum condition and parameters of growth.
Measurement the diameter and thickness of the biocellulose produced
The biocellulose was produced using the optimum conditions for the growth (20 g/L glucose, 4.75 pH and 30oC). The period of fermentation carried out for 8 days, and the sample was taken each day were each flask present day and the day next. The sample was removed by using tweezers, and treated the boiling in 0.1 NaOH, after that, the diameter measured by using listed ruler (mm) and the thickness measured by using Sylvac Digital Micrometer. The thickness was taken for the biocellulose pellicles in wet state and after the pellicle dried in the oven until the weight didn’t change.
Figure 4.7 shows the change in the thickness and the diameter of the biocellulose pellicles for 8 days of the fermentation. From Figure 4.5, it was observed that there is obvious increasing in the thickness of the cellulose pellicles along with the time, where the maximum thickness reached to 0.76 mm in the dry state and 8.351 mm in the wet state. And the diameter of the cellulose product as shown in Figure 4.5, there is no significant change in the diameter where the diameter was about 80 mm after one day from the fermentation and after four days of fermentation became 82 mm and continued to be constant until the end of fermentation. It is important to be noticed that the diameter depends on the diameter of the flask was used, because the cellulose product will be equal to the diameter surface of the medium as shown in the figure 4.8
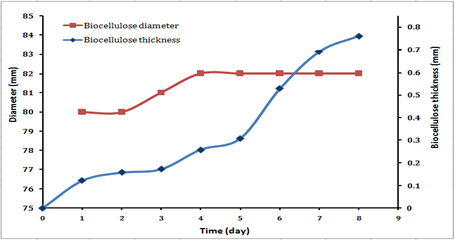
Figure 4.7 Thicknesses and Diameter of Biocellulose Pellicles

figure 4.8 biocellulose formed on the surface medium with diameter equal to surface medium
Measurement of the water holding capacity, and moisture content
The experiment carried out to determine the water holding capacity and moisture content of the samples was measured using the shake method (Schrecker et al 2005). eight samples of biocellulose were collected from 8 conical flasks 250 ml using tweezers and the samples was taken every day, where each flask represents the day and the day next. the pellicle of cellulose was allowed to drip for 30 sec and the wet weight was determined (Watanabe, K et al 1995). the moisture content (% w/w) of the cellulose was determined based on the weight loss of the cellulose when dried at 70 oc for 12 h under atmospheric pressure.
the water holding capacity was calculated using the following formula:
the moisture content is calculated by using this formula
 table 2.2 shows that (whc) and moisture content obtained for the biocellulose produced from the coconut medium. the samples that used to analyze the whc and the moisture content was conducted using experiment at optimum conditions (20 g/l glucose, 4.75 initial ph and autoclaved at the optimum temperature 30 oc). where 8 samples were used, and collected every day for 8 days of incubation. the moisture content of cellulose for the samples in the experiment was in the region of 92–96% and whc varied from 11% to 74%. the results showed that the biocellulose is about 4.5% of the total weight.
table 2.2 shows that (whc) and moisture content obtained for the biocellulose produced from the coconut medium. the samples that used to analyze the whc and the moisture content was conducted using experiment at optimum conditions (20 g/l glucose, 4.75 initial ph and autoclaved at the optimum temperature 30 oc). where 8 samples were used, and collected every day for 8 days of incubation. the moisture content of cellulose for the samples in the experiment was in the region of 92–96% and whc varied from 11% to 74%. the results showed that the biocellulose is about 4.5% of the total weight.
Table 2.2 whc and moisture content of the biocellulose production
| time (day) | wet weight (g/l ) | biocellulose
( g/l ) |
moisture content (%) | water holding capacity (%) |
| 0 | 0.00000 | 0.0000 | 0.000 | 0.000 |
| 1 | 18.5370 | 0.5670 | 96.94 | 31.69 |
| 2 | 154.183 | 2.0370 | 98.67 | 74.69 |
| 3 | 207.101 | 6.3070 | 96.95 | 31.83 |
| 4 | 210.557 | 10.026 | 95.23 | 20.00 |
| 5 | 275.064 | 11.662 | 95.76 | 22.58 |
| 6 | 288.961 | 20.082 | 93.05 | 13.38 |
| 7 | 332.506 | 25.686 | 92.27 | 11.94 |
| 8 | 386.500 | 27.096 | 92.98 | 13.26 |
Mcanning electron microscopy (SEM) analysis
The surface and cross-section of the samples were sputtered with gold and photographed. the biocellulose membranes were biosynthesized in the coconut media from static fermentation. The microfibrilar structure of microbial cellulose under the scanning electron microscope (SEM) is shown in Figure 4.7. The samples were chosen for the SEM from the experiment conducted by using the optimum conditions (20 g/L glucose, pH 4.75 and 30oC). 8 samples were collected for SEM; each sample was taken after each day from the incubation for 8 days.
Figure 4.9 presents typical SEM images of freeze-dried biocellulose and oven dried biocellulose composite. As seen from Fig. 4.7, biocellulose nanofibrils can be observed on the surface. The mean diameter of the nanofibrils is about 60 nm. From cross sectional images it was observed that these multiple layers structure have high aspect ratio. Evidently, the biocellulose sample has porous morphology, and these micrographs clearly show the various morphological features of these sheets. All samples showed a reticulated structure consisting of ultra-fine cellulose. Jaehwan (Jaehwan Kim et al 2010) reported that the biocellulose, which is synthesized by Acetobacter xylinum, consists of the biogenous nanofiber network structure formed by self-assembling in an efficient way. The cellulose is crystallized outward the organisms, particularly in Acetobacter xylinum that synthesizes cellulose chains by introducing glucose units to the reducing ends of the polymer. The growth mechanism during bacterial activity determines the morphology of the final cellulose.
Figure 4.10 present SEM images of the oven dried biocellulose; the biocellulose was dried at 70 oC until the weight remained constant. Through the images from figure 4.8, it was noted that the surface morphology of biocellulose was changed. Biocellulose nanofibrils cannot be observed due to the heat dryer. It is likely completely destroyed by the dry heat, which led to a shift to of an opaque surface. From the comparison between the images after the oven and freeze dry, there is a clear distinction and significant difference between the images after the oven dry and the freeze dry. Based on that, to keep and protect the biological structure and composition of biocellulose fibers, the process of drying by the freeze dryer is more efficient. Freeze drying was proved to be a more considerate drying method relating to the maintenance of pore structures (Klemm, D et al 2001).
During freeze drying the frozen water is removed by sublimation, thus reducing damage to biological structures. However, the level of cell viability after freeze drying varies according to numerous factors including the strain of microorganisms and also the efficacy of the protective agents used. Several investigators have reported the effect of bacterial cell size on survival during freeze drying and subsequent storage. Differences in the surface areas of microorganisms and variations in cell wall and membrane composition affect the ability of the cells to survive the freeze drying process (A. Jagannath et al 2010 ).
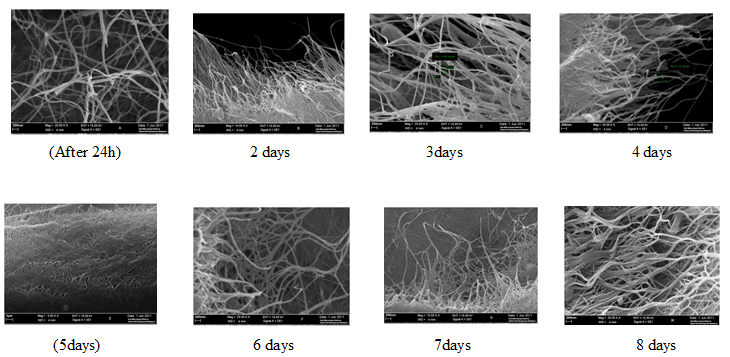
Figure 4.9 SEM images of freeze-dried biocellulose produced from a static culture from the fermentation of coconut medium. The biocellulose collected through 8 days from the fermentation, where the image (A) represent day 1 and the images B, C, D, E, F, G, and H represent days 2, 3, 4, 5, 6, 7 and 8 respectively.
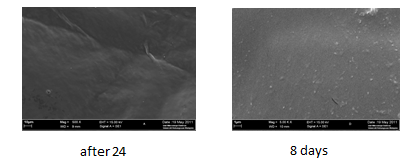
Figure 4.10 SEM images of oven dried biocellulose produced from a static culture from coconut medium. Where image (A) represent the biocellulose after 24h and (B) after 8 days from the fermentation.
5 – CONCLUSION
The findings from this study have shown that Acetobacter xylinum has the ability to produce biocellulose pellicles in the coconut medium. The results showed that the sugar concentrations (glucose), pH and temperature affected the yield of biocellulose significantly. The maximum biocellulose was produced by adding 20 g/L glucose to the coconut medium and the yield of biocellulose after 8 days from the fermentation was 27.096 gram of dry cellulose per liter of liquid medium. Among the three initial pH values studied (pH 4.0, 5.0 and 6.0), pH 4.0 was the best for bacterial growth and biocellulose production because it gave the highest yield of 26.139 g/L. The production of biocellulose has been investigated at different temperatures 25, 30, 35, 40oC. The highest yield of biocellulose was 26.036 g/L at 30oC. The optimum temperature was 30 oC for the production of biocellulose.
In addition, biocellulose has been characterized in order to evaluate the properties and the characteristics of the biocellulose. The characteristics of biocellulose considered were: thickness, diameter, water holding capacity and moisture content, besides that, SEM was taken in order to investigate the structure and the morphology of the cellulose. Characterization of biocellulose was conducted during the experiment and was carried out under the optimum conditions (20g/L glucose concentration, pH 4.75 and 30oC). Samples were taken each day for 8 days. The thickness of the biocellulose pellicles varied from 0.1215 – 0.760 mm in the dry state and from 1.509 – 8.351 mm in wet state. Also, it was observed that the thickness has a direct effect on the amount of water that biocellulose can hold which in turn affects the softness of the final product. Biocellulose has high and significant water holding capacity, where the WHC was varied from 11% – 74%. The biocellulose is about 4.5% of the total weight. The moisture content of biocellulose was in the range of 92–96% and the diameter of the pellicles was varied from 80-82 mm which was depends on the flask diameter.
The SEM photographs showed the structure of biocellulose when the samples were dried by the oven at 70oC until a constant weight was obtained; the surface morphology of biocellulose changed and biocellulose nanofibrils could not be observed due to the heat dryer. It might have been destroyed by the dry heat, which led to a shift to an opaque surface. While the biocellulose nanofibrils could be observed on the surface and the mean diameter of the nanofibrils is about 60 nm. Furthermore, all samples showed a reticulated structure consisting of ultra-fine cellulose when the samples were dried by freeze-dryer at −40 °C. Based on that, to keep and protect the biological structure and composition of biocellulose fibers, it is thus recommended that drying should be through the freeze dryer.
6 – REFERENCES
- Milda, E. Embuscado., Jay, S. Marks. & James, N. BeMiller. 1994. Bacterial cellulose Factors affecting the production of cellulose by Acetobacter xylinum. Food Hydrocolloids Vol.8 No.S pp.4l9-430 (1994), Department of Food Science, Purdue University, West Lafayette.
- F.D.E. Goelzer, P.C.S. Faria-Tischer, J.C. Vitorino, Maria, R. Sierakowski & C.A. Tischer. 2008. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Journal of Materials Science and Engineering C 29: 546–551
- Ross, P., Mayer, R. & Benziman, M. 1991. Cellulose biosynthesis and function in bacterial. Journal of Microbiological Reviews 55(1): 35-58
- Barbara Surma-Ślusarska, Sebastian Presler & Dariusz Danielewicz. 2008. Characteristics of Bacterial Cellulose Obtained from Acetobacter Xylinum Culture for Application in Papermaking. FIBRES & TEXTILES in Eastern Europe 2008, Vol. 16, No. 4 (69) pp. 108-111
- Angkana Phunsri, Pramote Tammarate, Warawut Krusong & Sumate Tantratian. 2003. The Liquid/Air Interface Area and Depth of Liquid Medium Suitable for cellulose production from Acetobacter TISTR 975. J. Sci. Res. Chula. Univ., Vol. 28, No. 1(2003).
- Shin Isawano, Kenji Tajima, Hiroyuiu Kono, Tomoki Erata, Masanobu Munekata & Mitsuo Takai. 2002. Effects of Endogenous Endo-P- 1,4-Glucanase on Cellulose Biosynthesis in Acetobacter xylinum ATCC23769. Journal of Bioscience Bioengineering Vol. 94, No. 3,275-28 1.2002
- Peter, G. verschuren., Thomas d. cardona., z m. j. robert nout., kees, d. de gooijer., & Johannes, c. van den heuvel. 2000. Location and limitation of cellulose production by Acetobacter xylinum established from oxygen profiles. Journal of biosciencaen d bioengineering Vol. 89, No. 5, 414-419
- Omer Shezad, Salman Khan, Taous Khan & Joong Kon Park. 2010. Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Journal of Carbohydrate Polymers 82: 173–180
- Angkana Phunsri, Pramote Tammarate, Warawut Krusong & Sumate Tantratian. 2003. The Liquid/Air Interface Area and Depth of Liquid Medium Suitable for cellulose production from Acetobacter TISTR 975. J. Sci. Res. Chula. Univ., Vol. 28, No. 1(2003).
- Schrecker, S. T. & Gostomski, P. A. 2005. Determining the water holding capacity of microbial cellulose. Journal of Biotechnology Letters, 27, 1435–1438
- Watanabe, K. & Yamanaka, S. 1995. Effects of oxygen-tension in the gaseous-phase on production and physical-properties of bacterial cellulose formed under static culture conditions. J. Biosci Biotechnol Biochem. 59:65–68
- young kook yang., sang hoon park., z jung wook hwang., yu ryang pyun. & yu sam kimit. 1998. Cellulose Production by Acetobacter xylinum BRCS under Agitated Condition. Journal of fermentation and bioengineering. Vol. 85, No. 3, 312-317
- Prashant R., Chawla Ishwar, B. B., Shrikant, A., Survase & Rekha S. Singhal. 2009. Microbial Cellulose: Fermentative Production and Applications. J. Fermentative Production of Microbial Cellulose, Food Technol. Biotechnol. 47 (2): 107–124.
- Masaoka, S., Ohe, T. & Sakota, N. 1993. Production of cellulose from glucose by Acetobacter xylinum, J. Ferment. Bioeng. 75(1), 18-22
- Schrecker, S. T. & Gostomski, P. A. 2005. Determining the water holding capacity of microbial cellulose. Journal of Biotechnology Letters, 27, 1435–1438
- Watanabe, K. & Yamanaka, S. 1995. Effects of oxygen-tension in the gaseous-phase on production and physical-properties of bacterial cellulose formed under static culture conditions. J. Biosci Biotechnol Biochem. 59:65–68
- Jaehwan Kim, Zhijiang Cai, Hyun Sook Lee, Gwang Seong Choi, Don Haeng Lee & Chulhee Jo. 2010. Preparation and characterization of a Bacterial cellulose/Chitosan composite for potential biomedical application. J. of Polym Res. DOI 10.1007/s10965-010-9470-9
- Klemm, D., Schumann, D., Udhart, U. & Marsch, S. 2001. Bacterial synthesized cellulose artificial blood vessels for microsurgery. Progress in polymer Science 26: 1561-1603
- A. Jagannath, P.S. Raju & A.S. Bawa. 2010. Comparative evaluation of bacterial cellulose (nata) as a cryoprotectant and carrier support during the freeze drying process of probiotic lactic acid bacteria. Journal of Food Science and Technology 43 (2010) 1197-1203
