Afra M. Yassin1
Doctor of philosophy (PhD), Science, Chemistry Department.
Email:Aframurtada27@gmail.com
HNSJ, 2022, 3(10); https://doi.org/10.53796/hnsj31025
Published at 01/10/2022 Accepted at 20/09/2022
Abstract
Amino acid complexes [M(L)2]Cl, [M(L)(Lʹ)]Cl, and [M(L)(Lʹ)(Lʹʹ)] type of Fe(III) ion with (Gly), and mixed amino acid ligands (Arg+Asp), and (Arg +Asp+Ser), respectively, have been synthesized and characterized using EDX, FTIR, UV/Vis, TGA, XRD, spectra and conductivity measurements. All amino acid ligands with Fe (III) ion have been found to act as bidentate chelating agents coordinating through COO– and NH2. The antibacterial activity of amino acid complexes was evaluated against four bacteria strains, two kind of grampositive (Staphylo coccus aureas, Klebsiella pneumonia), and two kind of gram negative (Escherichia coli, Pseudomonas aeruginosa). The Fe (III) complexes were found to have varied degree of inhibitory effect against bacteria.
Key Words: Iron, complex, antibacterial, amino acid, Escherichia coli, Klebsiella pneumoni, Staphylococcus aureas, Enterococcus feacalis
Introduction
The microbial resistance represent a problem and the outlook for the use of antimicrobial drugs in the future is still uncertain. Therefore, it must be taken measures to reduce this problem, for example, to control the use of antibiotic, develop research to better understand the genetic mechanisms of resistance, and to continue studies to develop new drugs, either synthetic or natural. The ultimate goal is to offer appropriate and efficient antimicrobial drugs to the patient.[1] For centuries, people have used cobalt and other ions to inhibit the growth of harmful microbes.
Coordination complexes of transition metals have been widely studied for their antibacterial, antifungal and potential cytotoxic chemotherapeutic agents. They have been evaluated against several pathogenic fungi and bacteria with promising results. One of the approaches to increases the efficacy of the drugs consists in their modification of physical and chemical factors. In addition to its ability to combat infection or neoplastic disease, these new agents must exhibit selective toxicity, chemical stability, and optimum rates of bio-transformation and elimination.[2]
Experimental
Material and methods
Chemical and reagent:
The chemicals used in the synthesis complexes are, iron (III) chloride hexa hydrate, sodium hydroxide, glycine, serine, arginine, aspartic acid, distill water and deionized water, absolute ethanol, formic acid.
Instruments used: IR spectrometer (FTIR), UV/Vis- Spectrometer, Conduct meter, Thermogravimetric analysis (TGA), X-Ray Diffraction (XRD), Energy dispersive X-ray (EDX).
Method
Iron amino acids synthesis
There were synthesized three amino acids complexes with iron (III) ions glycine, mixed ligand of (arginine+aspartic acid) and (arginine+aspartic acid+serine), (Fe-Gly, Fe-(Arg+Asp), Fe-(Arg+Asp+Ser), respectively. The solid complexes, [M(L)2]Cl, [M(L)(Lʹ)]Cl, and [M(L)(Lʹ)(Lʹʹ)] type of Fe(III) ion were prepared following the procedure described in the literature,[3] 1:2, 1:1:1, and 1:1:1:1 molar ratio, respectively, (aqueous solution) of metal chlorides and ligands (glycine, serine, arginine, aspartic acid), were mixed under stirring and heated under reflux for about one hour, the PH of solution was adjusted to about 8-10 using sodium hydroxide. As a result of the complex formation process the acidity of the reaction mixtures reached PH 4-6 and the color change. The reaction product was cooled, filtered, dried and kept in a desiccator over anhydrous CaCl2.
Physical Measurements
The conductance was measured in formic acid (10–3 M) on ELICO digital conductivity meter at room temperature, EDX analysis were recorded by using LEOS430 scanning, electron microscope coupled with energy dispersive X-ray analyzer model Oxford LINK ISIS, TGA was carried out on a Perkin– Elmer model TGS-2 instrument in temperature range (0-500ºC), IR spectra were recorded on Fourier-Transform (FT.IR) Spectrophotometer, Tensor 27 Co. Brucker 2003 at a range (400-4000 cm-1) using KBr discs, Electronic spectra were recorded on a UV-Vis. Spectrophotometer (Shimadzu, UV-1650PCb Spectrophotomete) using formic acid as a solvent at room temperature, and X-ray diffraction data were recorded on Philips PW 3710 diffractometer attached to digitized computer along with graphical assembly in which radiation source was connected with the tube Cu-K α ,25 Kv/20 mA. The scan range was between 3 and 80º 2θ.
Microbial strains
Standard strains of microorganism used in this study and were obtained from The National Health Laboratory and Management of laboratories, Khartoum. The bacteria species used were Escherichia coli, Klebsiella pneumonia, Staphylococcus aureas, Enterococcus feacalis. Bacteria were grown in Mueller Hinton Agar.
Antibacterial assay
Antibacterial activity of Fe3+ amino acid complexes was evaluated by disc diffusion method (Kil et al., 2009). Ni2+ amino acid complexes solution (100 mg/ml) stock solution were prepared by diluting with 5% formic acid. The test microorganisms were seeded into respective medium by spread plate method. After solidification, filter paper discs with a diameter of 6.0 mm were impregnated with 10, 20, 30, 40 μl of Fe3+ amino acid complexes, separately, followed by drying off. Formic acid was used as a negative control, while gentamicin (10μg/disc) was used as a positive control. Antibacterial discs were dispensed onto the surface of the inoculated agar plates and Petri plates were incubated for 24 h at 37°C. Diameters of clear zone of inhibition produced around the discs were measured and recorded.
Result and Discussion:
Physical analysis:
Molar conductivity of the dithiocarbamate complexes were measured in dimetylformamide (10-3M) solvent indicate that theses complexes are 1:1 electrolyte [8]. The corresponding Fe(Gly), Fe(Arg+Asp), and Fe(Arg+Asp+Ser) complexes, are soluble in formic acid but insoluble in THF, cyanomethane, benzene, dichloromethane, chloroform, DMSO, DMF+DMSO mixture and ethanol. Accordingly, the conductivity of the three complexes, were measured in 20% formic acid and the results obtain, indicate that Fe (Gly), and Fe (Arg+Asp) are 1:1 electrolytic nature, and the Fe (Arg+Asp+Ser) complex is unelectrolyte. Indicate that all ions involved in complex formation were coordinated with Ni2+ ion, in the other word, two groups coordinated by negative charge and the other groups coordinated by lone pair electrons.
Energy dispersive x-ray spectra (PCEDX) study:
The PCEDX profile of Fe(Gly), Fe(Arg+Asp), and Fe(Arg+Asp+Ser) complexes confirmed the presence of O, Co, C, N. the prominent nitrogen and oxygen clearly suggests to the functional group of amino acid ligand and Fe peak presence indicated to formation of complexes. These results had been summarized in table (1), (2), and (3) for Fe(Gly) The number of atoms was calculated according to their percentages shown by the apparatus where Fe(Gly) complex contains 4, 6, 1, 1 and 2 ions of C, O, Fe, Cl and N, respectively. Fe(Arg+Asp) complex contains 9, 7, 1, 1 and 5 ions of C, O, Fe, Cl and N, respectively In addition, Fe(Arg+Asp+Ser) complex contains 9, 7, 1, and 6 ions of C, O, Fe and N, respectively.
Table (1) explain PCEDX reading of Fe(Gly) complex
| δ3 | Unit | Result | Element |
| 0.127 | % | 35.428 | O |
| 0.446 | % | 20.610 | Fe |
| 0.175 | % | 17.710 | C |
| 0.210 | % | 13.100 | Cl |
| 0.245 | % | 10.332 | N |
| 0.320 | % | 2.825 | Trace element |
Table (2) explain PCEDX reading of Fe(Arg+Asp)complex
| δ3 | Unit | Result | Element |
| 0.149 | % | 30.381 | O |
| 0.253 | % | 28.426 | C |
| 0.037 | % | 16.572 | Fe |
| 0.101 | % | 13.218 | N |
| 0.099 | % | 8.386 | Cl |
| 0.320 | % | 3.064 | Trace element |
Table (3) explain PCEDX reading of Fe(Arg+Asp+Ser) complex
| δ3 | Unit | Result | Element |
| 0.164 | % | 33.6 | C |
| 0.120 | % | 31.013 | O |
| 0.210 | % | 18.077 | N |
| 0.135 | % | 12.03 | Fe |
| 0.320 | % | 5.265 | Trace element |
Thermal Analysis
Thermal Gravimetric Analysis (TGA) study for Fe (Gly), Fe (Arg+Asp), and Fe (Arg+Asp+Ser) complexes were investigated and recorded weight loss per mg against the temperature. The TGA curves of Fe.Gly, and mixed ligand Fe (Arg.Asp), showed same characteristic band in little difference in temperature and weight loss. TGA curve for three complexes displays two stages of mass loss within the temperature range of 100–500 °C. The first stage is at 100–180 °C, corresponding to the dehydration of two mols of crystal water. The second stage occurs at around 200–320 °C, corresponding to the loss of the volatile gases from decomposition of amino acid ligand. And the weight of the two complexes have been constant until the temperature reach 500°C, that indicate the Fe ion has not oxidation or decomposition occur. But the TGA curve for Fe(Arg.Asp.Ser) complex displays one stage of mass loss within the temperature range of 200–320 °C, corresponding to the loss of the volatile gases from decomposition of amino acid ligand. These feature summarized in table (4).
Table (4) explain weight loss per mg against to the temperture per (°C) reading Fe(Gly), Fe(Arg+Asp), and Fe(Arg+Asp+Ser) complexes:
| Fe(Arg+Asp+Ser) | Fe(Arg+Asp) | Fe(Gly) | |||
| Weight loss(mg) | Temp(°C) | Weight loss(mg) | Temp(°C) | Weight loss(mg) | Temp(°C) |
| 4.87 | 210 | 4.89 | 129 | 4.78 | 124 |
| 2.36 | 315 | 4.52 | 173 | 4.18 | 175 |
| 4.28 | 227 | 3.98 | 224 | ||
| 2.79 | 248 | 2.59 | 272 | ||
| 2.47 | 264 | ||||
Spectral studies:
IR-Spectra:
IR spectra were performed using [FT-IR] [ABB-MB 3000] spectrophotometer in the in the range (4000-400) cm-1 spectra were recorded as potassium bromide discs. The IR spectra for Fe(Gly), Fe(Arg+Asp), and Fe(Arg+Asp+Ser) complexes were showed a difference between the vibrational frequencies Vas (Coo-) at 1600 Cm-1 and Vs (Coo-) at 1400 Cm-1, generally increase from the theoretical values of free amino acid when the M-o bond strength depending on the carboxylate coordination[5].Two very well resolved bands at 1500 cm-1 and 1610 cm-1 for Vs and Vas of bending vibration and broad peak at 3100 cm-1 for stretching vibration are an indication of the amino group to the metal ion[6]. Infrared spectra of the complexes were also measured in the region 400-700 cm-1 in order to identify frequencies related to M-O and M-N bands. The M-O frequencies were identified at rang (600 –800) cm-1. While M-N frequencies were identified at rang (400 – 600) cm-1. These results are in agreement with literature value, being similar to other metal complexes with amino acid [7]. The v (O-H) stretching vibration do appear in the complexes at range (3450 – 3750) cm-1 suggesting the presence of the crystal and coordinated water in these compounds. These feature absolutely different in glycine, serine, aspartic acid, and arginine spectra. Which were summarized in table (5).
Table (5) important peaks appeared in the IR-Spectra of Glycine, Serine, Aspartic acid, and Arginine ligand and Co(Gly), Co(Ser), Co(Arg), and Co(Asp) complexes:
| Compound | COO- | NH3+ | M-O | M-N | O-H | |||
| Vasy | Vsy | Str(broad) | Bendasy | Bendsy | ||||
| Fe.Gly | 1631.67 | 1386.30 | 3417.63 | 1533.30 | 1466.50 | 673.11 | 428.17 | 3730.07 |
| Fe.Arg.Asp | 1645.17 | 1398.30 | 3419.56 | 1514.02 | 1427.23 | 516.89 | 441.67 | 3743.57 |
| Co.Arg.Asp.Ser | 1643.24 | 1425.30 | 3417.63 | 1626.90 | 1512.09 | 572.82 | 418.52 | 3741.65 |
UV/Vis spectra:
Fe(III) has the electronic configuration of d5, The ground state of d5 ion, 6S transforms into 6A1g – a singlet state. It is not split by the effect of crystal field and hence all the transitions are spin forbidden and are of less intensity. ii) In excited state, d5 ion gives rise to quartets (4G, 4F, 4D, 4P) and doublets (2I, 2H, 2G, 2F, 2D, 2P, 2S) . The transitions from the ground to doublet state are forbidden because the spin multiplicity 4T2g. The transitions which are independent of Dq and which result in sharp bands are 6A1g→ 4E (4D) 6A1g→ 4Eg+4E1g etc., iii). And some absorption bands are observed. Which are attributed to charge transfer from the non-bonding orbitals of the oxygen atoms in the ligand to the iron (III) d changes by two and hence they are too weak. Thus sextet-quartet forbidden transitions observed are: 6A1g→ 4T1g and 6A1g→ orbitals, And absorption bands are assigned to the ![]() →
→ ![]() * and n →
* and n → ![]() * transitions of the ligand [13],[14]. Then a sharp absorption bands appeared in the UV/Vis spectra, for Fe(Gly), Fe(Arg)(Asp), and Fe(Arg)(Asp)(Ser) complexes.
* transitions of the ligand [13],[14]. Then a sharp absorption bands appeared in the UV/Vis spectra, for Fe(Gly), Fe(Arg)(Asp), and Fe(Arg)(Asp)(Ser) complexes.
X-ray Diffraction (XRD) study:
X-ray diffraction patterns for three Iron-amino acid complexes (Fe(Gly), Fe(Arg)(Asp), and Fe(Arg)(Asp)(Ser) complexes) were recorded and calculated parameters are given in table (6) all complexes had a monoclinic crystal lattice.
Table (6) explain information recorded and calculated from XRD study
| Fe(Gly) | Fe(Arg)(Asp) | Fe(Arg)(Asp)(Ser) | ||
| Molecular formula | C4 H12 Fe O6 N2 Cl | C10 H25 Fe O8 N5 Cl | C13 H26 Fe O9 N6 | |
| Crystal system | monoclinic | monoclinic | monoclinic | |
| Unit cell | a | 5.191 A | 7.871 A | 9.898 A |
| b | 8.748 A | 9.978 A | 10.543 A | |
| c | 14.448 A | 18.448 A | 19.492 A | |
| α | – | – | – | |
| β | 90.81° | 94.81° | 93.81° | |
| δ | – | – | – | |
| Molecular weight | 275.35 | 434.35 | 465.84 | |
| Density per g/cm3 | 2.680 | 4.227 | 4.5336 | |
Biological Studies:
The antibacterial activity of amino acids complexes was investigated against isolated gram positive strain (Staphylococcus aureas and Enterococcus feacalis), and gram negative (Escherichia coli, Klebsiella pneumonia). And take some commercial antibiotics sensitivity as standard drug, which were amoxyclav, gentamicin, cefotaxime, vancomycin, ciprofloxacin, co-trimoxazole, ceftriaxone, and ampicillin. The results presented in Table (7) and figure (1), showed various degrees in antibiotic resistance. All bacterial species showed resistance to amoxyclav, cefotaxime, vancomycin (except S. aureus), and ampicillin. Among the tested bacterial isolates, the strongest antibacterial activities of antibiotics were obtained by vancomycin and gentamicin against S. aureus, with inhibition zones of 22 and 19 mm, respectively, (Saif et al., 2017). In Sudan, plant-based traditional medicine represents primary health care like extracts from Capparis decidua L. twigs (Abdalrahman et al., 2016). In this study found the Antibacterial effects of twigs extracts showed different degrees of inhibition profiles against tested bacteria. The ethyl acetate extract showed the highest activity against S. aureus (21 mm), B. subtilis (20 mm) and P. pneumoniae (18 mm) while the n-butanol extract displayed best inhibition against P. pneumoniae (18 mm) and E. coli (16 mm). All extracts showed high antifungal activity against A. niger and C. albicans with inhibition zone ranged from 17 to 22 mm. The antibacterial activity of amino acids complexes was investigated against isolated gram positive strain (Staphylo coccus aureas, Klebsiella pneumonia), and two kind of gram negative (Escherichia coli, Pseudomonas aeruginosa). The Fe(III) complexes of amino acid have inhibitory effect against gram positive and gram negative. The sensitivity of these complexes were determined the diameters of zone occurred by mean value (X′) of four difference concentrations (5 mg/weight) of complex. And evaluated the sensitivity of complexes according to chart (6mm ≡ R) (7-10mm ≡ SS), (10-20mm ≡ MS), and (<20mm ≡ HS), this illustrated in table (8), figure (2).
Table (7): Antibacterial sensitivity pattern of the test bacteria against 8 antibiotics using disc diffusion technique
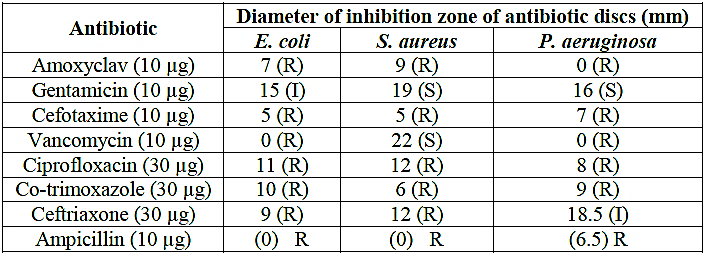
S, sensitive; R, resistant; I, intermediate
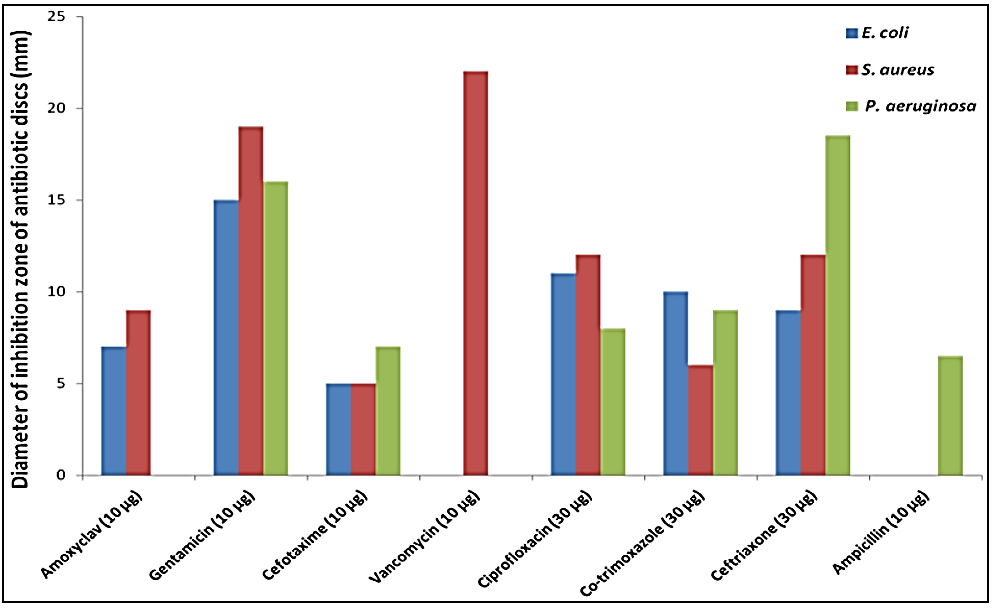
Figure (1): Antibacterial sensitivity pattern of the test bacteria against 8 antibiotics using disc diffusion technique
Table (8) Diameter ± SD and sensitivity of 5mg for Fe.Gly, Fe.Arg.Asp, and Fe.Arg.Asp.Ser complex against four types of bacteria:
| complex
Type of bacteria |
Fe.Gly | Fe.Arg.Asp | Arg.Asp.Ser | |||
| X´ ± SD | S(Sensitivity) | X´ ± SD | S(Sensitivity) | X´ ± SD | S(Sensitivity) | |
| Escherichia coli | 14 ± 0.707 | MS | – | R | – | R |
| Staphylo coccus aure | 18 ± 0.00 | MS | – | R | 9 ± 0.00 | SS |
| Pseudomonas aeruginosa | 19 ± 0.00 | MS | 15 ± 0.6324 | MS | 19 ± 1.0723 | MS |
| Klebsiella pneumonia | 15.5 ± 1.449 | MS | – | R | – | R |
Figure (2): Antibacterial sensitivity pattern of the test bacteria against Fe3+ complexes using disc diffusion techniqueConclusion:
Three amino acid complexes with Fe(III) ion [M(L)2]Cl, [M(L)(Lʹ)]Cl, and [M(L)(Lʹ)(Lʹʹ)] type of Fe(III) ion with (Gly), and mixed amino acid ligands (Arg+Asp), and (Arg +Asp+Ser), respectively were synthesized, by 1:2, 1:1:1, and 1:1:1:1 molar ratio in basic media, and characterized by PCEDX, TGA, FTIR, UV/Vis, XRD, and electrical conductivity measurement. Thermal gravimetric analysis of Fe.Gly and Fe.Arg.Asp complexes showed weight loss between 110-180°C, which equivalent to two moles of crystal water that agreement with result obtained from IR, PCEDX, and XRD analysis.
The Electrical conductivity of the complexes can provide us information about the number of ions involvement in the complexes in solution, which can give us a clear image about the structural geometry of the complexes; the electrical conductivity measurements were recorded of 2 mg/ml of complex solutions. The Fe.Gly and Fe.Arg.Asp complexes are electrolyte solution, 1:1 molar ratio this indicated that coordination to Fe (III) through the two COO- and two NH2 groups, and one mole of chloride ion containing out of the coordination sphere, and Fe.Arg.Asp.Ser complex is non-electrolyte solution, (neutral complex) this indicated that coordination to Fe through the two COO- and two NH2 groups, this proposal was supported by the IR-spectra, PCEDX, and XRD analysis of the complexes. As a result as the complexes form are [Fe(gly)2.(H2O)2]Cl (a), [Fe(arg)(asp).(H2O)2]Cl (b), and [Co(arg)(asp)(ser)] (c), Which illustrated in sketch (1) bellow:
| (a) | (b) | (c) |
Sketch (1): explain structure of [Fe(Gly)2.(H2O)2]Cl (a), [Fe(Arg)(Asp).(H2O)2]Cl, (b) [Fe(Arg)(Asp)(Ser)] (c), complexes
Refrence:
1. Kabbani AT, Hammud HH, Ghannoum AM (2007). Preparation and antibacterial activity of copper and cobalt complexes of 4-chloro-3-nitrobenzoate with a nitrogen donor ligand. Chem Pharm Bull 55(3):446-450.
2. Johari R, Kumar G, Kumar D, Singh S (2009). Synthesis and antibacterial activity of M (II) Schiff-Base complex. J Ind Council Chem 26(1):23-27.
3. Stanila A, Marcu A, Rusu D, Rusu M, David L (2007). Spectroscopic studies of some copper (II) complexes with amino acids. J Mol Struct 834:364-368.
4. Pal S, Kyung TY, Myong SJ (2007). Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia col. Appl Environ Microbiol 73(6):1712-1720.
5. S Nagae; M Ushijima; S Hatono; J Imai; S Kasuga; H Matsuura; Y Itakura; Y Higashi. Planta Med. 1994, 60(3),214-217.
6. P Mejnikov; P Corbi; C Aguila. J. Alloys comp. 2000, 307(1-2), 179-183.
7. C Batiu; C Jelic; N Leopold; O Cozar; L David, J. Mol. Struct. 2005, 325-330, 744-747.
