Alia.z.hashim1; Raqad R.Al-Hatim2; Faleeha Hasan Hussein3; Zena Kadhim Al-younis4
Department of Food Science, College of Agriculture, University of Basrah, Iraq
Corresponding author: alia.hashim@uobasrah.edu.iq
HNSJ, 2023, 4(3); https://doi.org/10.53796/hnsj4321
Published at 01/03/2023 Accepted at 12/02/2023
Abstract
Microalgae are a large diverse group of microorganisms comprising photoautotrophic protists and prokaryotic cyanobacteria—also called as blue-green algae. These microalgae form the source of the food chain for more than 70% of the world’s biomass. It contains higher nutritional values, with rapid growth characteristics. Microalgae are autotrophic organisms and extensively desired for use in nutraceuticals and as supplement in diet. Many microalgae species are documented for health benefits, by strengthening immune system and by increasing the nutritional constitution of body. Many countries have been experienced an increase in protein consumption over the last decades due to the population growth and adoption of protein-rich dietaries. Unfortunately, conventional-based protein agroindustry is associated with environmental impacts that might aggravate as the humankind increase. Thus, screening for novel protein sources that are environmentally friendly may contribute to conventional agriculture to provide food and feed in a more sustainable manner. In this context, microalgae farming are a promising alternative to couple the anthropic emissions with the production of food and feed. Some strains show protein contents two times higher than conventional protein sources produced from animals and plants. The use of whole microalgae biomass as a protein source in food and feed is simple and well-established. Conversely, the production of microalgae protein supplements and isolates requires the development of feasible and robust processes able to fractionate the microalgae biomass in different value-added products.
Key Words: Microalgae, Functional properties food, Nutritional value
عنوان البحث
القيمة الغذائية والخصائص الوظيفية لبروتينات الطحالب
عالية زيارة هاشم1، رغد رحيم حاتم1، فليحه حسن حسين1، زينة كاظم اليونس1
1 قسم علوم الاغذية ، كلية الزراعة ، جامعة البصرة ، العراق
بريد الكتروني: alia.hashim@uobasrah.edu.iq
HNSJ, 2023, 4(3); https://doi.org/10.53796/hnsj4321
تاريخ النشر: 01/03/2023م تاريخ القبول: 12/02/2023م
المستخلص
الطحالب الدقيقة هي مجموعة كبيرة ومتنوعة من الكائنات الحية الدقيقة التي تتكون من الطحالب ذات التغذية الضوئية والبكتيريا الزرقاء بدائية النواة – وتسمى أيضًا الطحالب الخضراء المزرقة. تشكل هذه الطحالب الدقيقة مصدر السلسلة الغذائية لأكثر من 70٪ من الكتلة الحيوية في العالم. يحتوي على قيم غذائية أعلى ، مع خصائص النمو السريع. الطحالب الدقيقة هي كائنات ذاتية التغذية ومطلوبة على نطاق واسع لاستخدامها في المغذيات وكمكمل في النظام الغذائي. تم توثيق العديد من أنواع الطحالب الدقيقة لفوائدها الصحية ، من خلال تقوية جهاز المناعة وزيادة التكوين الغذائي للجسم. شهدت العديد من البلدان زيادة في استهلاك البروتين على مدى العقود الماضية بسبب النمو السكاني واعتماد الأنظمة الغذائية الغنية بالبروتين. لسوء الحظ ، ترتبط الصناعة الزراعية التقليدية للبروتين بالآثار البيئية التي قد تتفاقم مع زيادة الجنس البشري. وبالتالي ، فإن فحص مصادر البروتين الجديدة الصديقة للبيئة قد يساهم في الزراعة التقليدية لتوفير الغذاء والأعلاف بطريقة أكثر استدامة. في هذا السياق ، تعد زراعة الطحالب الدقيقة بديلاً واعدًا لمزاوجة الانبعاثات البشرية مع إنتاج الغذاء والأعلاف. تظهر بعض السلالات محتوى بروتين أعلى مرتين من مصادر البروتين التقليدية المنتجة من الحيوانات والنباتات. يعد استخدام الكتلة الحيوية للطحالب الدقيقة الكاملة كمصدر للبروتين في الغذاء والأعلاف أمرًا بسيطًا وراسخًا. وعلى العكس من ذلك ، فإن إنتاج مكملات وعزل بروتين الطحالب الدقيقة يتطلب تطوير عمليات مجدية وقوية قادرة على تجزئة الكتلة الحيوية للطحالب الدقيقة في منتجات مختلفة ذات قيمة مضافة.
الكلمات المفتاحية: الطحالب الدقيقة ، الخصائص الوظيفية للغذاء ، القيمة الغذائية
Introduction
The total protein content of microalgae biomass varies depending on the kind of microalgae and can reach 70% dry weight (Schade et al., 2020; Amorim et al., 2020). Microalgae cell walls are frequently damaged in order to gain access to proteins, amino acids, and other components. Some microalgae have been found to have soluble proteins in their cytoplasm (Chew et al., 2019; El-Nagger et al., 2020). Soluble protein, central pyrenoid, and phytobiliproteins are also seen in microalgae with chloroplasts, while other microalgae, such as Arthrospira platensis, have thylakoid sacs enclosing the peripheral cytoplasm associated with phycobilisomes (Bishop and Zubeck, 2012; Dolganyuk et al., 2020). In recent years, the number of studies on methods of digesting microalgae and exploiting them as a source of protein has exploded (Ghribi et al., 2015; Vernèsa et al., 2019).
1-Species of Algae
The term “algae” refers to a large range of eukaryotic organisms, usually chlorophyll-containing, that are either grown or wild collected and come from a variety of watery settings. Algae are thought to be one of the oldest forms of life. So far, between 40,000 and 100,000 species of algae have been identified, albeit this figure may be conservative (Hu et al., 2008). Most algae, like terrestrial plants, are photoautotrophs, meaning they can fix inorganic carbon from the atmosphere and turn sunlight into chemical energy through photosynthesis (6CO2 + 6H2O + light energy C6H12O6 (sugars) + 6O2). Photosynthesis produces sugars, which are then transformed into other biological components like lipids, carbohydrates, and proteins, which make up biomass , Certain algae, on the other hand, are heterotrophs, meaning they use an organic carbon substrate (such as glucose, acetate, and fructose) as their only carbon and energy source while converting it to chemical energy. Under dark conditions, this procedure entails the employment of fermenters that are supplied with oxygen for algae metabolic growth (also known as aerobic respiration). Furthermore, certain organisms are mixotrophic, meaning they may gather energy for growth through both phototrophic and heterotrophic processes while consuming both inorganic CO2 and organic carbon substrates (Darzins. et al., 2010). In comparison to photoautotrophic and heterotrophic cultures, certain species (such as the blue-green microalgae Spirulina) develop at their fastest in mixotrophic culture, which uses light and glucose simultaneously. A more in-depth look at algae biomass culture techniques in varied growth settings, including photoautotrophic, heterotrophic, and mixotrophic environments (Chojnacka and Noworyta, 2004).
2-Composition of Bio chemicals
The cellular composition of each fraction changes depending on the specific strain of alga and their physiological responses to biotic and abiotic variables, such as light intensity, photoperiod, temperature, nutrients, and development phase. ) Barkia et al.,2019) In reality, these parameters influence not only photosynthesis and cell biomass productivity, but also the pattern, pathway, and activity of cellular metabolism, altering cell composition as a result (Patil et al.,2005) As a result of their evolutionary and phylogenetic diversity, as well as the ability to manipulate cultivation settings to drive chemical production, these microorganisms have become particularly appealing for bioprospecting and commercial exploitation of a wide range of biomolecules (Gouveia et al., 2010; Borowitzka,2013). Microalgae bioactive substances can be obtained directly from primary metabolism, such as proteins, fatty acids, and vitamins, or created from secondary metabolism. Such compounds may have a variety of biological actions that could be useful in the treatment and prevention of diseases bioactive chemicals are accumulated in the biomass of most microalgae; however, in some situations, these metabolites are ejected into the medium and are referred to as exometabolites (De Morais et al., 2015). Proteins/enzymes, lipids/fatty acids, carbohydrates, pigments, vitamins, minerals, and additional substances not covered in these classifications can all be found in microalgae (Jacob-Lopes et al.,2019). Despite the biochemical differences amongst microalgae classes and species, protein is often the most abundant organic ingredient (12–35%), followed by lipids (7–23%) and carbs (5–23%). However, as previously indicated ((Patil et al., 2005),
1-2 Protein
Spirulina has very high protein content, ranging from 55 to 70% by dry weight, depending on the source (Phang et al., 2000). It is a complete protein, having all essential amino acids but with lower quantities of methionine, Cystine, and lysine than standard proteins like meat, eggs, or milk; yet, it outperforms all typical plant proteins like those found in legumes.
2-2 Essential fatty acids
Polyunsaturated fatty acids (PUFAs) account for 1.5–2.0 percent of total lipid in spirulina, making it a good source of essential fatty acids. Spirulina is particularly high in -linolenic acid (ALA), linoleic acid (LA, 36 percent of total), stearidonic acid (SDA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (AA). (Haug et al., 2011).
3-2 Vitamins
Vitamin B1 (thiamine), B2 (riboflavin), B3 (nicotinamide), B6 (pyridoxine), B9 (folic acid), B12 (cyanocobalamin), vitamin C, vitamin D, and vitamin E are all found in spirulina.
4-2 Minerals
Spirulina is high in potassium and contains other minerals such as calcium, chromium, copper, iron, magnesium, manganese, phosphorus, selenium, sodium, and zinc.
5-2 pigments
One of the most obvious and arresting characteristic of the algae is their colour. In general, each phylum has its own particular combination of pigments and an individual colour. Aside chlorophylls, as the primary photosynthetic pigment, microalgae also form various accessory or secondary pigments, such as phycobiliproteins and a wide range of carotenoids. These natural pigments are able to improve the efficiency of light energy utilization of the algae and protect them against solar radiation and related effects. Their function as antioxidants in the plant shows interesting parallels with their potential role as antioxidants in foods and humans (Van den Berg et al., 2000).Spirulina cultivated under laboratory circumstances, gathered in natural conditions, and in mass culture systems using various agroindustrial waste effluents have all been subjected to detailed biochemical composition analysis. This was discovered to fluctuate in response to the salinity of the growing media — salt-adapted cells exhibited a modified biochemical composition with lower protein and chlorophyll content and higher carbohydrate content, according to Vonshak et al. (1996). However, the following is a summary of the literature on spirulina’s broad makeup.
3- Microalgae proteins
Microalgae proteins can be found in a variety of sites, including the cell wall, cytoplasm, organelles, plastids, and nucleus (Safi et al., 2015).Microalgae have a protein content ranging from 20% to 70%, depending on the species and growing conditions, and an amino acid profile that is suitable for human consumption (Becker, 2007; Khatoon et al., 2014; Menegol et al., 2017; Seyfabadi et al., 2011; Teuling et al., 2017; Waghmare et al., 2016). The cyanobacterium Arthrospira platensis and the green microalgae Haematococcus pluvialis, for example, have 6,630 and 18,545 protein-coding genes, respectively, whereas strains have 9,349 to 10,240. (Luo et al., 2019; Wu et al., 2019). Surprisingly, the number of protein-coding genes is not directly related to protein content, and microalgae like Arthrospira and Chlorella (El-Kassas et al., 2015). To measure the protein concentration, the total nitrogen content of the microalgae biomass is normally multiplied by a nitrogen-to-protein conversion factor (N-Prot factor). The N-Prot factor of 6.25 is based on the assumption that the samples contain 16 percent (w/w) nitrogen-containing proteins with very little non-protein nitrogen (NPN).
Table 1. Compares the general composition of several green microalgae to various food protein sources (w/w percent dry matter) (Becker, 1994, Belitz et al., 2009; Schwenzfeier et al., 2011)
| Commodity | Proteins | Lipid | Carbohydrate |
| Tetraselmis sp. | 36 | 22 | 42 |
| Chlamydomonas rheinhardii | 48 | 21 | 17 |
| Chlorella vulgaris | 55 | 18 | 15 |
| Dunaliella salina | 57 | 6 | 32 |
| Scendesmus obliquus | 53 | 13 | 14 |
| Egg white | 88 | < 1 | 17 |
| Milk | 26 | 28 | 38 |
| Soybean | 37 | 20 | 30 |
4-Nutritional quality of microalgae proteins
The assessment of “protein quality” is one of the most important criteria for evaluating novel food and feed resources for their nutritional value. Protein quality is a set of metrics used to assess a dietary protein source’s (or dietary mixes’) ability to meet the metabolic needs of a target animal for amino acids and nitrogen (e.g., maintenance, growth, tissue repair, reproduction, lactation). This is mostly due to the amino acid content, digestibility, and bioavailability of the protein source, as well as the consuming animal’s daily dietary nitrogenous requirements (Boye et al., 2012). For about 60 years, protein quality in microalgae has been studied by food scientists and applied dietitians. The first studies on Arthrospira (Spirulina), Chlorella, Scenedesmus, and Spongiococcum began in the 1950s with the aim of determining their potential as superfoods for the rising worldwide human population as well as a compact, nutrient-dense sustenance for planned space missions (Becker, 2013).
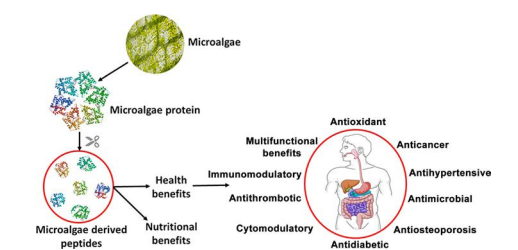
Figure 1. Nutritional and health benefits of proteins and peptides derived from microalgae (Acquah et al., 2020)
1-4 Quality of microalgae protein for nutrition
The assessment of the amino acid profile is an important criterion for evaluating the protein quality. Essential amino acids are given special attention because they are not manufactured by the human body and must be received through food (Damodaran et al., 2007). Isoleucine, leucine, valine, lysine, phenylalanine, tyrosine, methionine, cysteine, tryptophan, threonine, and histidine are necessary amino acids. The UN’s Food and Agriculture Organization (FAO/WHO, 2007) developed a reference standard that included the recommended quantity of essential amino acids in a protein or a protein mixture. The amino acid profiles of eggs and soybeans revealed appropriate levels of all necessary amino acids, whereas the Cyanobacterium aphanizomenon sp. had inadequate levels. When microalgae are cultured under different conditions, they produce distinct amino acid profiles (James et al., 1989), hence optimizing microalgae growth conditions could lead to the creation of proteins with the right amino acid profile.
2-4 Microalgae amino acid profile
While the overall protein concentration of microalgae varies widely in the literature, ranging from 7% to 70% (Becker, 2007; Laurens et al., 2012), the amino acid profile of microalgae protein is rather consistent, regardless of algae species or growth strategy. Some of the 20 amino acids that make up the body’s proteins are regarded as either non-essential (dispensable) or essential (necessary) in terms of nutrition (indispensable). Nonessential amino acids (NEAAs) can be produced from scratch in large enough quantities to meet daily protein demands. Essential amino acids (EAAs), on the other hand, cannot be produced endogenously in sufficient quantities to meet daily protein demands and must be obtained through the diet. Some amino acids are considered “dietary necessary” because the consumer lacks the biochemical mechanisms needed to manufacture the chemical configurations of the carbon chain skeletons of those amino acids (Jobling, 1994). Arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine are required in the diets of most monogastric species (e.g., chicken, swine, fish, and rodents). With the exception of key developmental stages, ill-health, and premature infants (Reeds, 2000; McDonald et al., 2010), arginine is not considered essential for humans. Although high protein content and a well-balanced EAA profile (as measured by the EAAI) may provide investigators and product developers with solid early indicators of microalgae protein quality, these factors alone do not guarantee high dietary protein consumption by consumers. There must be a mix of biochemical studies, EAA profiles, protein and amino acid bio accessibility, and biological evaluations to determine the efficiency of protein utilization. Many microalgae, in particular, have an extra nutritional barrier that may be absent in other traditional protein sources. Many microalgae have refractory cell walls, and digestibility is the initial barrier for optimal protein digestion by humans and animals after consumption (Boye et al., 2012).
3-4 Amino Acid Composition
Protein quality can vary substantially depending on digestibility and critical amino acid availability (Boisen and Eggum, 1991). Animal sources of protein are often regarded as complete proteins because they contain a high concentration of essential amino acids (EAAs) that the human body cannot produce. Plant proteins, on the other hand, are frequently regarded as inadequate protein sources because they lack one or more important amino acids, such as histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine (Young and Pellett, 1994). However, the missing necessary amino acid (s) in plant-based proteins can vary, which means that if a person eats a varied diet of plant proteins from fruits, vegetables, grains, and legumes, they should be able to get enough of all essential amino acids. Due to their high concentration of insoluble polysaccharides, plant-based proteins are often more difficult to digest than animal proteins. Despite this, concerns about the high levels of saturated fats and cholesterol found in animal-based meals, which have been related to the development of cardiovascular disease and diabetes, are growing. As a result, nutritionists and organizations like the FAO have begun to advocate for a more diversified diet rich in plant-based proteins (FAO, 1991). Algae are generally considered a suitable protein source since their EAA composition meets FAO requirements and they are often comparable to other protein sources like soybeans and eggs (Fleurence, 1991). The lack of widespread ingestion of marine algae has resulted in a scarcity of in vivo studies on ileal digestion, restricting comparisons of algae protein quality between different species.AAS (amino acid score) = mg of amino acid per gram of test protein divided by mg of the same amino acid per gram of reference protein (Godfray et al.,2010) Following that, PDCAAS was used to correct AAS for the test protein’s real fecal protein digestibility as assessed in a rat assay, which was computed as follows: PDCAAS (protein digestibility corrected AAS) = fecal true digestibility (%) = (mg of limiting amino acid per gram of test protein/mg of same amino acid per gram of reference protein).
4-4 Algae Protein Digestibility
The fraction of ingested dietary components that is available at the target site of action for use in various physiological activities is known as bioavailability (Guerra et al., 2012). Bioavailability refers to the entire process that occurs after a food element is consumed, including digestibility and solubility in the gastrointestinal tract, absorption/assimilation across intestinal epithelial cells and into the circulatory system, and finally incorporation into the target site of utilisation (Figure 1) (Ekmekcioglu, 2002). In vivo tests must therefore be included in studies assessing the bioavailability of dietary components. One in vivo investigation looked at the bioavailability of P. tenera and U. pinnatifida in Wistar rats and found that seaweed fiber reduced protein intake digestibility and meal efficiency (Urbano and Goñi, 2002). Similarly, L. japonica has been shown to reduce protein digestibility in rats, albeit, curiously, after 3 weeks, digestibility was comparable to the control diet, indicating that the rats had adapted to the high fiber diet (Suzuki et al.,1993). Phlorotannins and high polysaccharide content are regarded to be the key factors that reduce the digestion of algae proteins (Wong and Cheung, 2001; Joubert and Fleurence, 2008).
5-4 Recovery of microalgae proteins
In open or closed culture methods, crude protein recovery from microalgae is typically done after biomass generation. Attempts to create microalgae strains capable of secreting recombinant proteins in the growth medium have recently yielded encouraging results (Lauersen et al., 2015; Baier et al., 2018). Microalgae strains developed for secretion of specific recombinant proteins, on the other hand, have a greater potential to produce biotechnological applications in niche markets, whereas algae farming based on the biorefinery for crude protein production and advanced biofuel production requires robust processes with easy scalabilities. To avoid the low digestion of algae protein, separate extracts and use them instead of the entire biomass (Grossmann et al., 2018; Schwenzfeier et al., 2013). Protein extracts can then improve algae protein digestibility by removing the cell wall, increasing the selling price as compared to the entire biomass (Bleakley and Hayes, 2017). Furthermore, high viscosity neutral or anionic cell-wall polysaccharides (e.g., agar, alginates, or carrageenans) might obstruct the extraction process as well as fractionation and recovery methods in seaweed. The recovery technique should be tailored to solve these concerns based on the characteristics and type of the algae wall. Disruption techniques are commonly used as a first step to facilitate membrane breaching and complete access to interior contents, thus making extraction in a specific solvent medium easier. Because the technique will directly affect protein bioactivity as well as bioavailability, technical functionality, and taste, the extraction parameters must be chosen according to the desired aim, Physical procedures, such as mechanical disintegration and non-mechanical extraction, have been used in previous protein extraction methods. In addition, innovative approaches such as ultrasonication, ohmic heating (OH), pulsed electric fields (PEF), and microwaves are already being employed to avoid some of the issues associated with traditional procedures (such as time consumption and protein integrity loss) (Bleakley and Hayes, 2017).
5- Microalgae toxicity
Because of the wide variety of microalgae species and the need to screen for prospective commercial strains, potential dangers in their use as food and feed must be carefully considered. The FDA (United States Food and Drug Administration) has classified Arthrospira, Chlorella, Dunaliella, Haematococcus, and Schizochytrium as “Generally Recognized as Safe” (Chacón-Lee and González- Mariño, 2010). However, a recent study found that microcystin levels in four of 18 dietary supplements containing Arthrospira and Aphanizomenon flos-aquae exceeded the World Health Organization’s acceptable daily intake threshold of 0.04 g.kg-1 bodyweight, Toxins in algae dietary supplements may be present for three reasons: (1) growth of toxin-producing microalgae such as A. flos-aquae DC-1 (Zhang et al. 2016); (2) infection of the microalga culture with a toxin-producing bacterium; and (3) utilization of toxin-containing water sources. To ensure the safety of these goods, thorough quality control, continual monitoring of algae-based supplements and the establishment of the maximum intake of distinct microalgae toxins are all necessary (Roy-Lachapelle et al. 2017). Toxin-producing microalgae are typically cyanobacteria or dinoflagellates (Qian et al., 2015; Carmichael and Boyer, 2016). Toxin-producing cyanobacteria and dinoflagellates develop quickly in the seas and rivers, killing marine animals and causing intoxication and death in birds and humans (Richmond and Hu 2013).
6- Protein Extraction
1-6 Cell Structures
Because most microalgae proteins are generated intracellularly, their extraction success is heavily dependent on their accessibility. As a result, the complexity of algae cell walls is the greatest obstacle to using seaweed as a protein source. Algae cells have a large number of intracellular enzymes and proteins (Maehre, 2015), their cell walls are made up of a complex network of biopolymers, primarily polysaccharides, that interact with water and metal cations, among other molecules (Domozych, 2019). The fibrillar wall, the amorphous matrix, and the glycoprotein domain are the three primary domains of the cell wall; the reticulated network of fibrillar polysaccharides and glycoproteins is embedded in the gel-like amorphous matrix. The fibrous section of the cell wall is the most chemically and physically resistant, with cellulose being the most important constituent among others like xylan and hemicellulose (Cian et al., 2015).
2-6 Extraction Methods
The cell wall is required for the extraction and usage of algae proteins. Additional stress variables are frequently used to assure intracellular protein extraction, which improves extraction efficiency. Algae proteins have yet to be fully described, so they are simply divided into four classes based on their solubility: albumins, which are soluble in water; globulins, which are soluble in salt solutions; glutelins, which are soluble in dilute acids or bases; and prolamins, which are soluble in 70% alcohol in water (Maehre, 2015). Unless a specific type of protein is being targeted, equential extraction procedures are frequently carried out to ensure the extraction of multiple types of proteins.
7-Extraction and purification of microalgae proteins
Unicellular microalgae like Tetraselmis sp. have strong cellulose cell walls that make removing intracellular proteins challenging (Becker, 2007; Doucha and Livansky, 2008). Disrupting the microalgae cells is the first step in protein extraction, and this can be done via high-pressure homogenization, milling, ultra-sonication, chemical or enzymatic lysis, or a combination of these methods (Phong et al., 2018; Safi et al., 2014; Soto-Sierra et al., 2018). The crude extract contains microalgae proteins as well as all other constituents such as lipids, carbohydrates, cell wall contents, vitamins, and salts. Recent approaches, such as therapy with a pulsed electric field (PEF), aim to remove microalgae contents more gently in order to maintain live cells (Buchmann et al., 2019; Luengo et al., 2014). Centrifugation can separate a water-soluble and an insoluble fraction from a crude microalgae extract (henceforth referred to as soluble or insoluble for convenience). Although the soluble fraction is free of larger pollutants and particles, it may still contain other soluble chemicals that interfere with protein function (Grossmann et al., 2018; Garcia et al., 2018a). Surfactants and charged polysaccharides, in particular, have an effect on the interfacial environment, as will be discussed further below. As a result, the soluble microalgae fraction is usually purified for proteins to generate a protein isolate. Precipitation of proteins (e.g. 10 or 7 percent Trichloroacetic acid (w/v) (TCA). Because most of the techniques were directed at improving protein accessibility for digestive enzymes or devised for analytical objectives, protein denaturation was not an issue in those studies. The isolates obtained were typically weak in solubility as a result of the harsh isolation procedures applied. One method for re-solubilizing precipitated proteins was to break them down into peptides with either NaOH at high temperatures or a proteolytic enzyme. To cover all possible technological and functional uses, microalgae proteins must be isolated as intact proteins in a soluble condition. Despite the fact that Meijer and Wijffels (1998) and Herrero et al. (2005) describe extraction processes that produce soluble protein fractions, the use of sodium dodecyl sulfate (SDS) and ammonium sulfate, respectively, is undesirable. SDS binds to the proteins in the solution, altering their technological and functional properties, particularly their interfacial properties. Ammonium sulfate precipitations are typically unsuitable for large-scale applications; hence, the most common and straightforward procedure is fractionation and resolubilization of the precipitate. The disadvantage of this method is that it only yields protein fractions with the same pI, leaving proteins with different (pI) behind (Grossmann et al., 2019). Protein re-solubility after precipitation may also be affected. Other techniques include protein precipitation with ammonium salts, organic solvents, or ionic liquids, albeit these require additional resources (Grossmann et al., 2018; Garcia et al., 2018b). Dialysis, ultrafiltration, or membrane filtration can be used to further purify the protein isolate (Safi et al., 2017; Garcia et al., 2018b). Microalgae proteins can therefore be separated gradually from crude extract to soluble fraction to yield a protein isolate, which can then be further processed to remove remaining contaminants.
8- Concentration of microalgae proteins
Dewatering may be required to concentrate microalgae proteins for the development of commercial applications such as protein concentrates and isolates. There are numerous protein concentration method, Trichloroacetic acid or ammonium sulfate are commonly used to precipitate proteins, although these chemicals remain in the protein concentrate, and their removal may be required for particular uses, such as food and feed (Pohanish, 2002; Gerde et al., 2013). Ultrafiltration is a protein concentration technology that does not require the addition of salt or acid. This method is based on the use of semipermeable membranes that allow tiny molecules (such as water and salts) to pass through while retaining bigger molecules like proteins (Cheryan, 1998). Freeze-drying, which involves eliminating water at low temperature and pressure by sublimation, is another chemical-free technology widely used in protein concentration (Gerde et al. 2013; Field et al. 2016). Freeze-drying improves protein durability by lowering residual water content, allowing freeze-dried proteins to be stored at room temperature with minimal protein activity loss (Kutz, 2007). However, before using the freeze-drying process, the salt level of the microalgae protein extract should be assessed. After the water is removed, freeze-drying raises the concentration of salts and a high salt content can modify the ionic strength and pH, which can denature proteins (Matejtschuk, 2007) (figure 2).
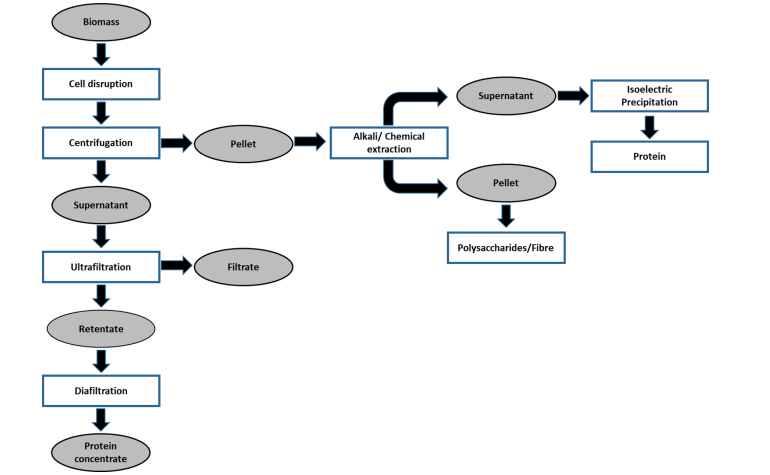
Figure 2. Bio-refinery based sequential protein extraction from microalgae biomass. (Siddiki et al., 2022)
9- Technological Functionality
Proteins have numerous purposes in foods that go beyond their nutritional value, such as the application of desirable qualities and their physical behavior during preparation, transformation, and storage, in addition to their fascinating impacts on human health. Micro-and macroalgae might thus play a key role through their structural biopolymers, which have both techno-and bio-functional uses (emulsification and foaming capabilities, for example) (Bernaerts et al., 2019).
1 -9 Solubility
One of the most important prerequisites for the successful use of protein isolates in meals is high solubility. A single protein’s solubility is often limited because the overall net charge is zero and electrostatic repulsion is low around the isoelectric point (pI). At all other pH values, electrostatic repulsion between individual protein molecules is high, so they are kept in solution as long as their native form is maintained (Fennema et al., 2008). The pH-dependent solubility behavior of a single protein usually results in a bell-shaped solubility curve with a minimum around pI. (Gonzalez-Perez et al., 2005; Lakemond et al., 2000). Several proteins, however, have great solubility even when their pI is near 0 (e.g., -lactoglobulin, ovalbumin, and sunflower albumins). However, after denaturation (e.g., by heat), the solubility of those proteins often falls towards the pI due to increased exposure of hydrophobic groups at the protein surface (Zhu and Damodaran, 1994). The solubility of a single protein can be affected by the presence of other molecules (e.g. other proteins or polysaccharides). Sunflower albumins (SFA) are very soluble in their purified form across a wide pH range. The electrostatic interactions between SFA and helianthinin (the two protei) are destroyed when both proteins are present in a full sunflower isolate. The two proteins are oppositely charged between pH values of 5.5 and 7.0, resulting in lower solubility between pH 4.0 and 5.5. In addition to pH, ionic strength (I) affects protein solubility. In general, it is assumed that a moderate increase in ionic strength promotes solubility. For glycinin, a 0.5 M ionic strength was shown to be sufficient to give high solubility (90%) around the pI (pH 5). However, raising the ionic strength to 0.25 M in the instance of helianthinin has been shown to reduce solubility at pH 5.
2-9 Emulsifications
Proteins are already employed as emulsifiers because they may stabilize the aqueous-organic/oily interface. Because many meals contain both lipidic and aqueous phases, this characteristic is critical. The maximum amount of oil that can be dispersed in an emulsifier solution without creating destabilization in its structure due to coalescence, creaming, flocculation, or sedimentation over a defined time period is defined as the emulsifying ability of proteins, also known as emulsifying capacity (EC) (Kumari et al., 2014). Changes in pH and ionic strength affect the development and stability of protein–polysaccharides owing to the presence of mainly electrostatic interactions (Schwenzfeier et al., 2012). The EC is often higher for higher pH values (7–10) and lower for lower pH values (3) due to the extraction/purification procedure used. Divalent cations (e.g., Ca2+) can also have a negative impact on the emulsion due to decreased electrostatic repulsion and ion bridging (Grossmann et al., 2020). However, because polysaccharides (such as uronic acid) can be utilized as emulsifiers, they can be coupled with proteins to produce advantageous and more stable emulsifying complexes that can be employed in food (such as gum Arabic) (Grossmann et al., 2018; Schwenzfeier et al., 2012).
3-9 Foaming
Protein foams are present in bread, cakes, cookies, meringues, ice creams, and other bakery products. They are made up of the dispersion of gases in a liquid or solid phase, and their amphiphilic behavior is linked to their appearance. Proteins have the unique capacity to generate an elastic and dense interfacial coating between the two phases of foams, allowing them to retain air and improve their favorable textural properties. Surface hydrophobicity, ligand binding, molecular flexibility, and protein structural stability all affect this feature, which is regulated directly by the extraction approach as well as the drying method (Grossmann et al., 2020). Furthermore, Benelhadj et al. (2016) found that protein foaming capabilities were impacted by pH variations and treatment time in a recent study. The foaming properties of a protein isolate isolated from Arthrospira platensis were found to be minimum at pH 3 and maximal at pH 10. At pH 10, the hydrophobic interaction was reduced, but protein flexibility was stronger, resulting in better foaming capabilities (Zheng et al., 2020). Furthermore, at pH 10, an improvement in foaming qualities was seen after 30 minutes of treatment, probably due to increased solubility and surface activity of the soluble proteins.
4-9 Gelation
The gelation mechanism and gel nature of protein gels are directly related to several factors, including (protein type and concentration, pH, ionic strength) reducing agents denaturants and miscible solvents, due to possible changes in protein native form, net charge, and electrostatic interactions. Bashir et al. (2016) studied the functional features of Spirulina platensis protein isolates and discovered that the isolates showed good gelling properties when compared to Spirulina cell suspension.
10- Antibacterial activity of microalgae
The antibacterial activity of microalgae is an undiscovered resource among the various niches potentially relevant to the pharmaceutical business. Although eukaryotic microalgae are known to produce bioactive chemicals, most published evidence on new antibacterial compounds comes from diatoms or cyanobacteria. The urgent need for novel antibacterial medicines is a well-recognized global concern due to growing bacterial resistance to antibiotics currently on the market. Colistin-resistant genes have been discovered in 19 countries since the end of 2015, when new mechanisms of resistance to colistin (the final line of antibacterial treatment) were discovered in Escherichia coli (Liu et al., 2015). According to O’Neill (2014) by 2050, antimicrobial resistance will be responsible for 10 million deaths, far more than cancer.
11- Antioxidants
Microalgae are photoautotrophic organisms that have evolved various effective defensive systems against reactive oxygen species and free radicals as a result of their high oxygen and radical stress (Pulz and Gross, 2004). As a result, there is growing interest in employing microalgae as a natural antioxidant source for cosmetics (such as sunblock) and functional foods/ nutraceuticals. According to Natrah et al. (2007), methanolic microalgae crude extracts (from e.g. Isochrysis galbana, Chlorella vulgaris, Nannochloropsis oculata, Tetraselmis tetrathele, and Chaetoceros calcitrans) had higher antioxidant activity than a-tocopherol but were lower than the synthetic antioxidant BHT. However, the safety of BHT and BHA synthetic antioxidants is debatable, as they are thought to be carcinogenic and tumorigenic when taken in large concentrations (Schildermann et al., 1995; Aruoma, 2003).
12-Food applications of microalgae
Microalgae powders, pills, capsules, and liquids have all been utilized as food (Spolaore et al. 2006; Brennan and Owende 2010; Wells et al. 2017). The health benefits of eating microalgae, such as anti-inflammatory, brain development, hypocholesterolemia, antioxidant, blood cell creation, and pro-vitamin A, are driving the production of these microorganisms as food (De Jesus Raposo et al., 2013; Wells et al. 2017). Furthermore, algae farming can be carried out in areas where the soil, water, or climates are unsuitable for conventional crops, thus allowing the production of food and energy in areas where people are hungry (FAO, 2009; FAO, 2011; Stamer, 2015). According to a recent study, protein-rich extracts of Chlorella protothecoides offer promising sensory qualities, which are required for meal absorption (Grossmann et al., 2019). Low protein dispersibility and the unpleasant taste profile of many alternative proteins such as pea, potato, or rice proteins at low pH is now a barrier in beverage composition (Grossmann et al., 2019). Other foods where microalgae have been successfully employed to improve nutritional or sensory characteristics include yogurt with A. plantensis and pasta with C. vulgaris and A. maxima (Barkallah et al., 2017). Furthermore, microalgae like Schizochytrium limancinum can be fed to dairy cows to boost the concentration of long-chain n-3 fatty acids in milk and cheese, providing further human health advantages (Till et al. 2019).
Figure 3. Functional properties and Anti activity algae protein
13- Applications in Food
Microalgae proteins can be used for feed in the form of complete algae biomass or as extracted proteins. Indeed, bioactive peptides from algae proteins may also be incorporated into feed products for health-beneficial bioactivities. Bioactive peptides are usually between 2 and 30 amino acids in length and following consumption may impart a health benefit to the consumer that goes above and beyond basic human nutrition. Using the whole biomass has advantages such as lower cost of production and additional nutrients. Most in vivo experiments performed to date with microalgae proteins as feed were performed with complete algae biomass, usually preprocessed to open the cells and thereby make the nutrients more accessible. Advantages of using extracted proteins include reducing the potential complications associated with the low digestibility of certain carbohydrates and lipids in some animals among others. Some general criteria that are needed if algae biomass is used as feed include, in addition to the nutrient content (1) acceptable nutrient availability for uptake, digestibility, palatability, and animal acceptance, (2) effect on weight gain and on total weight, feed incorporation properties, absence of toxins, and effects on appearance, and (3) the taste and smell of the finished product. In addition, for lactating cows, the production efficiency and nutrient content of milk are essential as foregg-producing hens. The most economically-valuable algae products are the macroalgae polysaccharides, like agar, alginates and carrageenans, especially due to their rheological gelling or thickening properties food (Spolaore et al., 2006). Polysaccharides are widely used in the food industry primarily as gelling and/or thickening agents. Many commercially used polysaccharides like agar, alginates and carrageenans are extracted from macroalgae (e.g. Laminaria, Gracilaria, Macrocystis) (Borowitzka, 1988). The most promising microalga for commercially purposes is the unicelular red algae Porphyridium cruentum, which produces a sulphated galactan exopolysaccharide that can replace carrageenans in many applications). Certain highly sulphated algae polysaccharides also present pharmacological properties acting on the stimulation of the human immune system (Pulz and Gross, 2004).
1-13 Food Gel
Most recently, our group is studying the incorporation microalgae biomass in food gelledproducts, based on protein and polysaccharide mixed biopolymer systems. Gelled vegetable desserts, alternative to dairy desserts, with pea protein isolate (4% w/w), k-carrageenan (0.15%) and starch (2.5%), optimized in previous studies (Nunes et al., 2003) were used as model systems. The gels were prepared with different microalgae – Spirulina (Arthrosphira) maxima, Chlorella vulgaris (green and orange, after carotenogenesis), Haematococcus pluvialis (red, carotenogenic) and Diacronema vlkianum – were evaluated in terms of colour and texture and compared with gels prepared with commercial pigments –phycocyanin, astaxanthin, b-carotene, canthaxanthin and lutein.
2-13 biscuits
A similar study was performed, this time using Isochrysis galbana biomass (Gouveia et al., 2008), that was cultivated in the Department of Aquaculture of IPIMAR (Portugal). Anenhancement of the biscuits texture properties and high stability of colour and texture along three months storage was observed, as previously reported for Chlorella biscuits (Gouveia et al., 2007). The biscuits presented quite different tonalities, turning from green to a brownish and duller tonality when increasing the biomass concentration from 1.0% to 3.0%. The main interest in using Isochrysis galbana biomass is due to its high levels of long chain omega-3 polyunsaturated fatty acids, especially EPA and DHA (Bandarra et al., 2003). The biscuits fatty acids profile is clearly related to butter (Özkanli and Kaya, 2007), with predominance of saturated (~60%) and monounsaturated fatty acids (~30%), mainly palmitic acid (30-40%) and oleic acid (18:1w9) (20-25%), respectively. Polyunsaturated fatty acids corresponded to 6-9% (4-5% linoleic acid; 18:2w6), the highest levels being for 3% Ig Biscuits (55% linoleic acid, 15% EPA, 6% _-linolenic acid and 3% DHA).
3-13 Biscuits Coloured
A study was undertaken to determine the effects of adding Chlorella vulgaris biomass as a colouring ingredient in traditional butter biscuits (Gouveia et al., 2007). The cookies were manufactured at a pilot scale, according to an optimized formulations from previous studies (Piteira et al., 2004), and stored for three months at room temperature, protected from light and air. Chlorella vulgaris biscuits presented an accentuated green tonality, which increased with the amount of added biomass. In general, colour parameters (CIELAB system) remained very stable along the storage period. However, it seems not necessarily to use biomass concentrations above 1% (w/w), since the green tonality.
References
Acquah, C.; Tibbetts, S. M.; Pan, S., and Udenigwe, C. (2020). Nutritional quality and bioactive properties of proteins and peptides from microalgae. In Handbook of Microalgae-Based Processes and Products, pp. 493-531. Academic Press.
Amorim, M.L.; Soares, J.; dos Reis Coimbra, J.S.; de Oliveira Leite, M.; Teixeira Albino, L.F.and Martins, M.A. (2020). Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr., pp. 1–27.
Aruoma, O.I. (2003). Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutation Research, 523, pp. 9-20.
Baier, T.; Kros, D.; Feiner, R. C.; Lauersen, K. J.; Müller, K. M., and Kruse, O. (2018). Engineered fusion proteins for efficient protein secretion and purification of a human growth factor from the green microalga Chlamydomonas reinhardtii. ACS synthetic biology, 7(11), pp. 2547-2557.
Bandarra, N.M.; Pereira, P.A.; Batista, I. and Vilela, M.H. (2003). Fatty acids, sterols and –tocopherol in Isochrysis galbana. Journal of Food Lipids, 18, pp. 25-34.
Barkallah, M.; Dammak, M.; Louati,I.; Hentati,F.; Hadrich,B.; Mechichi,T.; Ayadi, M.A.; Fendri, I.; Attia, H. and Abdelkafi, S. (2017). “Effect of Spirulina Platensis Fortification on Physicochemical, Textural, Antioxidant and Sensory Properties of Yogurt during Fermentation and Storage.” LWT 84 (October): pp. 323–330.
Barkia, I.; Saari, N. and Manning, S.R. (2019). Microalgae for high-value products towards human health and nutrition.Mar. Drugs 17, pp. 1–29.
Bashir, S.; Sharif, M.K.; Butt, M.S.and Shahid, M. (2016). Functional properties and amino acid profile of Spirulina platensis protein isolates. Biological Sciences-PJSIR, 59(1), pp. 12–19.
Becker, E.W.(1994). Microalgae: Biotechnology and Microbiology. Cambridge University Press, Cambridge, UK
Becker,E.W. (2007). “Micro-Algae as a Source of Protein.” Biotechnology Advances 25 (2), pp. 207–210.
Becker, E.W. (2013). Microalgae for human and animal nutrition. In: Handbook of Microalgal Culture. John Wiley and Sons, Oxford, UK.
Belitz, H.D.; Grosch, W. and Schieberle, P. (2009). Food Chemistry. Springer, Berlin, Germany.
Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H. and Ghorbel, D. (2016). Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 194, pp. 1056–1063.
Bernaerts, T.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E. and Van Loey, A.M. (2019). The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnology advances, 37(8), 107419.
Bishop, W.M. and Zubeck, H.M. (2012).Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J. Nutr. Food Sci.2 (5), pp. 1-6.
Bleakley, S. and Hayes, M. (2017). Algal proteins: extraction, application, and challenges concerning production. Foods, 6(5), pp. 33.
Boisen, S. and Eggum, B. (1991). Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr. Res. Rev., 4, pp. 141–162.
Borowitzka, M.A. (1988). Vitamins and fine chemicals from micro-algae. In M.A. Borowitzka, and L.J. Borowitzka (Eds), Micro-algal biotechnology (pp. 153-196). Cambridge, UK: Cambridge University Press.
Borowitzka, M. A. (2013). High-value products from microalgae-their development and commercialisation. Journal of Applied Phycology, 25(3),pp. 743–756.
Boye, J.; Wijesinha-Bettoni, R. and Burlingame, B. (2012). Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. British Journal of Nutrition, 108 (S2), S183–S211.
Brennan, L. and Owende, P. (2010). “Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products.” Renewable and Sustainable Energy Reviews 14 (2), pp.557–577.
Buchmann, L.; Bertsch, P.; B¨ocker, L.; Kr¨ahenmann, U.; Fischer, P. and Mathys, A. (2019). Adsorption kinetics and foaming properties of soluble microalgae fractions at the air/water interface. Food Hydrocolloids, 97, 105182.
Carmichael, W. W., and Boyer, G.L. (2016). “Health Impacts from Cyanobacteria Harmful Algae Blooms: Implications for the North American Great Lakes.” Harmful Algae 54, pp.194–212.
Chacón-Lee, T.L. and G.E. González-Mariño. (2010). “Microalgae for ‘Healthy’ Foods -Possibilities and Challenges.” Comprehensive Reviews in Food Science and Food Safety 9 (6), 655–675.
Cheryan, M.. (1998). Ultrafiltration and Microfiltration Handbook. 2nd ed. CRC Press.
Chew K.W.; Chia, S.R.; Lee, S.Y.; Zhu, L. and Show, P.L. (2019) Enhanced microalgal protein extraction and purification using sustainable microwave-assisted multiphase partitioning technique. Chem. Eng. J., 367, pp.1–8.
Chojnacka, K. and Noworyta, A. (2004). “Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures,” Enzyme and microbial technology, 34(5), pp. 461-465.
Cian, R.; Drago, S.; de Medina, F. and Martínez-Augustin, O. (2015). Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs, 13, pp.5358–5383.
Damodaran, S.; Parkin, K. L. and Fennema, O. R. (2007). Fennema’s food chemistry. 4th ed. CRC Press.
Darzins, A.; Pienkos, P. and Edye, L. (2010).”Current status and potential for algal biofuels production,” A report to IEA Bioenergy Task, 39, pp. 403-412.
De Jesus Raposo, M. F.; de Morais, R. M. S. C. and de Morais, A. M. M. B. (2013). Health applications of bioactive compounds from marine microalgae. Life sciences, 93(15), pp. 479-486.
De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.and Costa, J.A.V.(2015). Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int, 15, pp.1–15.
Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N. and Sukhikh, S.(2020). Microalgae: A promising source of valuable bioproducts. Biomolecules 10(8), 1153.
Domozych, D. (2019). “Algal cell walls” in eLS (John Wiley and Sons, , Ltd:Chichester,https://doi.org/10.1002/9780470015902.a0000315.pub4., pp. 11.
Doucha, J.and Livansky, K. (2008). Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Applied Microbiology and Biotechnology, 81(3), pp.431-440.
Ekmekcioglu, C. (2002).Aphysiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem., 76(2), pp. 225–230.
El-Kassas, H. Y., Heneash, A. M., and Hussein, N. R. (2015). Cultivation of Arthrospira (Spirulina) platensis using confectionary wastes for aquaculture feeding. Journal of Genetic Engineering and Biotechnology, 13(2), 145-155. Academy of Scientific Research and Technology, pp.145–155.
El-Naggar, N. E. A.; Hussein, M. H.; Shaaban-Dessuuki, S. A. and Dalal, S. R. (2020). Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Scientific Reports, 10(1), 3011.
FAO: Food and Agriculture Organization. (1991). Protein Quality Evaluation—Report of Joint FAO/WHO Expert Consultation; FAO: Rome, Italy.
FAO. (2009). How to Feed the World in 2050. Rome.
FAO. (2011). World Livestock – Livestock in Food Security. Rome.
Fennema, O.R.; Damodaran, S. and Parkin, K.L. (2008). Fennema’s food chemistry. CRC, Boca Raton, FL, USA.
Field, L. M; Fagerberg,W.R. Gatto, K.K. and Anne Böttger, S. (2016). “A Comparison of Protein Extraction Methods Optimizing High Protein Yields from Marine Algae and Cyanobacteria.” Journal of Applied Phycology, December. Journal of Applied Phycology, 29, pp. 1271-1278.
Fleurence, J. (1991). Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol., 10, pp. 25–28.
Garcia, E. S.; van Leeuwen, J.; Safi, C.; Sijtsma, L.; Eppink, M.H.; Wijffels, R.H. and van den Berg, C. (2018). Selective and energy efficient extraction of functional proteins from microalgae for food applications. Bioresource Technology, 268, 197–203.
Garcia, E. S.; Ruiz, C. A. S.; Tilaye, T.; Eppink, M. H.; Wijffels, R. H. and van den Berg, C. (2018). Fractionation of proteins and carbohydrates from crude microalgae extracts using an ionic liquid based-aqueous two phase system. Separation and Purification Technology, 204, pp.56–65.
Gerde, J. A.; Wang, T.; Yao, L.; Jung, S.; Johnson, L. A. and Lamsal, B. (2013). “Optimizing protein isolation from defatted and non-defatted Nannochloropsis microalgae biomass.” Algal Research, 2 (2), pp.145–153.
Ghribi, A.M.; Gafsi, I.M.; Blecker, C.; Danthine, S.; Attia, H. and Besbes, S.(2015). Effect of drying methods on physicochemical and functional properties of chickpea protein concentrates. Journal of Food Engineering, 165, pp. 179–188.
Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M. and Toulmin, C.(2010). Food security: The challenge of feeding 9 billion people. Science, 327(5967), pp.812–818.
Gonzalez-Perez, S.; Vereijken, J.M.; Van Koningsveld, G.A.; Gruppen, H.and Voragen, A.G.J. (2005). Physicochemical properties of 2S Albumins and the corresponding protein isolate from sunflower (Helianthus annuus). Journal of Food Science, 70(1), C98-C103.
Gouveia, L., Batista, A.P., Miranda, A., Empis, J., and Raymundo, A. (2007). Chlorella vulgaris biomass used as colouring source in traditional butter cookies. Innovative Food Science and Emerging Technologies, 8(3), pp.433-436.
Gouveia, L., Coutinho, C., Mendonça, E., Batista, A.P., Sousa, I., Bandarra, N.M., and Raymundo, A. (2008). Sweet biscuits with Isochrysis galbana microalga biomass as a functional ingredient. Journal of the Science of Food and Agriculture, 88, pp.891-896.
Gouveia, L.; Marques, A.E.; Sousa, J.M.; Moura, P. and Bandarra, N.M.(2010). Microalgae–source of natural bioactive molecules as functional ingredients. Food Sci. Technol Bull Funct Foods 7(2),PP. 21–37.
Grossmann, L.; Ebert, S.; Hinrichs, J. and Weiss, J. (2018). Effect of precipitation, lyophilization, and organic solvent extraction on preparation of protein-rich powders from the microalgae Chlorella protothecoides. Algal Research, 29, PP. 266–276.
Grossmann, L.; Ebert, S.; Hinrichs, J. and Weiss, J. (2019). Formation and stability of emulsions prepared with a water-soluble extract from the microalga Chlorella protothecoides. Journal of Agricultural and Food Chemistry, 67, pp.6551–6558.
Grossmann, L.; Hinrichs, J. and Weiss, J. (2019). Solubility of extracted proteins from Chlorella sorokiniana, Phaeodactylum tricornutum, and Nannochloropsis oceanica: Impact of pH-value. LWT, 105, pp. 408–416.
Grossmann, L.; Wörner,V.; Hinrichs, J. and Weiss,J. (2019). “Sensory Properties of Aqueous Dispersions of Protein-Rich Extracts from Chlorella Protothecoides at Neutral and Acidic PH.” Journal of the Science of Food and Agriculture, 100(3), pp.1344-1349.
Grossmann, L.; Hinrichs, J. and Weiss, J., (2020). Cultivation and downstream processing of microalgae and cyanobacteria to generate protein-based techno functional food ingredients. Critical Reviews in Food Science and Nutrition, 60(17), pp.2961–2989.
Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S. and Alric, M. (2012). Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol., 30, pp.591–600.
Haug, I.J.; Sagmo L.B.; Zeiss, D.; Olsen, I.C.; Draget, K.I. and Seternes, T. (2011).Bioavailability of EPA and DHA delivered by gelled emulsions and soft gel capsules. European Journal of Lipid Science and Technology, 113(2), pp.137–145.
Herrero, M.; Martin-Alvarez, P.J.; Senorans, F.J.; Cifuentes, A. and Ibanez, E. (2005). Optimization ofaccelerated solvent extraction of antioxidants from Spirulinaplatensismicroalga. FoodChemistry, 93(3), pp.417-423.
Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M. and Darzins, A. (2008). “Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances,” The Plant Journal, vol. 54(4), pp. 621-639.
Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R. and Zepka, L.Q. (2019). Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Current Opinion in Food Science, 25, pp.1–7.
James, C.M.; Al-Hinty, S. and Salman, A.E. (1989). “Growth and Ω3 fatty acid and amino acid composition of microalgae under different temperature regimes.” Aquaculture 77 (4), pp.337–351.
Jobling, M. (1994). Fish Bioenergetics. Chapman and Hall, New York, NY, p. 309. https://www.springer.com/gp/ book/9780412580901.84 (4), pp.1879–1887.
Joubert, Y.and Fleurence, J. (2008).Simultaneous extraction of proteins and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). Journal of Applied phycology, 20, pp.55–61.
Khatoon, H.; Rahman, N.A.; Banerjee, S.; Harun, N.; Suleiman, S. S.; Zakaria, N. H.; Lananan, F.; Abdul Hamid, S. H. and Endut, A. (2014). Effects of different salinities and pH on the growth and proximate composition of Nannochloropsis sp. and Tetraselmis sp. isolated from South China Sea cultured under control and natural condition. International Biodeterioration and Biodegradation, 95,pp. 11-18.
Kumari, P.; Kumar, M.; Reddy, C.R.K.and Jha, B., (2014). Nitrate and phosphate regimes induced lipidomic and biochemical changes in the intertidal macroalga Ulvalactuca (ulvophyceae, chlorophyta). Plant Cell Physiology.55 (1), pp.52–63.
Kutz, M. (Ed.) (2007). “Food Drying and Evaporation Processing Operations.” In Handbook of Farm, Dairy, and Food Machinery, William Andrew, 303–340.
Lakemond, C.M.M.; De Jongh, H.H.J.; Hessing, M.; Gruppen, H. and Voragen, A.G.J. (2000). Soy glycinin:Influence of pH and ionic strength on solubility and molecular structure at ambient temperatures.Journal of Agricultural and Food Chemistry, 48(6), pp.1985-1990.
Lauersen, K. J.; Huber, I.; Wichmann, J. ; Baier, T.; Leiter, A.; Gaukel, V. Viktor Kartushin, et al. (2015). “Investigating the Dynamics of Recombinant Protein Secretion from a microalgal host.” Journal of Biotechnology 215, pp.62–71.
Laurens, L.M.L.; Dempster, T.A.; Jones, H.D.T.; Wolfrum, E.J.; Van Wychen, S.; McAllister, J.S.P.; Rencenberger, M.; Parchert, K.J. and Gloe, L.M. (2012). Algal biomass constituent analysis: method uncertainties and investigation of the underlying measuring chemistries. Anal. Chem.
Liu, Y. Y.; Wang, Y.; Walsh, T.R.; Yi, L. X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; Yu, L. F.; Gu, D.; Ren, H.; Chen, X.; Lv, L.; He, D.; Zhou, H.; Liang, Z.; Liu, J. H. and Shen, J.(2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: microbiological and molecular biological study. The Lancet infectious diseases, 16, (2), pp.161–168.
Luengo, E.; Cond´on-Abanto, S.; ´Alvarez, I. and Raso, J. (2014). Effect of pulsed electric field treatments on permeabilization and extraction of pigments from Chlorella vulgaris. Journal of Membrane Biology, 247, pp.1269–1277.
Luo, Q.; Bian, C.; Tao, M.; Huang, Y.; Zheng, Y.; Lv, Y.; … and Hu, Z. (2019). Genome and transcriptome sequencing of the astaxanthin-producing green microalga, Haematococcus pluvialis. Genome biology and evolution, 11(1), pp.166-173.
Maehre, H. (2015). Seaweed Proteins-How to Get to Them? Effects of Processing on Nutritional Value, Bioaccessibility and Extractability. Ph.D. Thesis, Faculty of Biosciences, Fisheries and Economics, Norwegian College of Fishery Science, Tromsø, Norway.
Matejtschuk,Paul. (2007).“Lyophilization of Proteins.” In Cryopreservation and Freeze-Drying Protocols, edited by John G. Day and Glyn N. Stacey, 2nd ed., pp.59–72.
McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Morgan, C.A.; Sinclair, L.A. and Wilkinson, R.G. (2010). In: McDonald, P. (Ed.), Animal Nutrition, seventh ed. Pearson, Harlow, UK.
Meijer, E.A. and Wijffels, R.H. (1998). Development of a fast, reproducible and effective method for the extraction and quantification of proteins of micro-algae. Biotechnology Techniques, 12(5), pp.353-358.
Menegol, T.; Diprat, A. B.; Rodrigues, E. and Rech, R. (2017). Effect of temperature and nitrogen concentration on biomass composition of Heterochlorella luteoviridis. Food Science and Technology, 37, pp.28–37.
Natrah, F.M.I.; Yosoff, F.M.; Shariff, M.; Abas, F. and Mariana, N.S. (2007). Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. Journal of Applied Phycology, 19, pp. 711-718.
Nunes, J. C., Bouaoune, Y., Delechelle, E., Niang, O., and Bunel, P. (2003). Image analysis by bidimensional empirical mode decomposition. Image and vision computing, 21(12), 1019-1026.
O’Neill, J.(2014). Review on Antibiotic Resistance Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Welcome Trust. Heal. Wealth Nations, PP. 1-16.
Özkanli, O. and Kaya, A. (2007). Storage stability of butter oils from sheep’s non pasteurized and pasteurized milk. Food Chemistry, 100, pp.1026-1031.
Patil, V.; Reitan, K.; Mortensen, L.; Källqvist, T.; Olsen, Y.; Vogt, G. and Gislerød, H. (2005).Microalgae as a source of polyunsaturated fatty acids for aquaculture. Plant Biol, 6(6), pp.57-65.
Phong, W. N.; Show, P. L.; Le, C. F.; Tao, Y.; Chang, J. S.and Ling, T. C. (2018). Improving cell disruption efficiency to facilitate protein release from microalgae using chemical and mechanical integrated method. Biochemical Engineering Journal, 135, pp.83–90.
Phang, S.M.; Miah, M.S.; Chu, W.L. and Hashim, M.A. (2000). Spirulina culture in digested sago starch factory waste water. Journal of Applied Phycology, 12, pp.395–400.
Piteira, F.; Nunes, M.C.; Raymundo, A. and Sousa, I. (2004). Effect of principal ingredients on quality of cookies with dietary fibre. In P.A. Williams, and G.O. Phillips (Eds), Gums and Stabilisers for the Food Industry 12 (pp. 475-483). Cambridge, UK: Royal Society of Chemistry.
Pohanish, R.P., ed. (2002). “Trichloroacetic Acid.” In Sittig’s Handbook of Toxic and Hazardous Chemicals and Carcinogens, 4th ed., 2, PP. 2239–2241. Norwich: William Andrew Publishing.
Pulz, O. and Gross, W. (2004). Valuable products from biotechnology of microalgae.Applied Microbiology and Biotechnology, 65,PP. 635-648.
Qian, Z.J.; Kang, K. H.; and Ryu, B. (2015). Chapter 35 – Microalgae-Derived Toxic Compounds. In Handbook of Marine Microalgae. In Handbook of marine microalgae pp.527-537. Academic Press.
Reeds, P.J., (2000). Dispensable and indispensable amino acids for humans. The Journal of Nutrition, 130 (7), 1835S–1840S.
Richmond, A. and Hu, Q. (2013). Handbook of Microalgal Culture: Applied Phycology and Biotechnology. Edited by Amos Richmond and Qiang Hu. John Wiley and Sons, Ltd.
Roy-Lachapelle, A.; Solliec, M.; Bouchard, M. and Sauvé, S. (2017). “Detection of Cyanotoxins in Algae Dietary Supplements.” Toxins 9 (3), 76.
Safi, C.; Ursu, A. V.;Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P. Y. and Vaca-Garcia, C. (2014). Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Research, 3, pp.61–65.
Safi, C.; Frances,C.; Ursu,A.V.; Laroche,C.; Pouzet,C.; Vaca-Garcia,C. and Pontalier,P. (2015). “Understanding the Effect of Cell Disruption Methods on the Diffusion of Chlorella Vulgaris Proteins and Pigments in the Aqueous Phase.” Algal Research 8, pp.61–68.
Safi, C.; Olivieri, G.; Campos, R. P.; Engelen-Smit, N.; Mulder, W. J.; van den Broek, L. A. and Sijtsma, L. (2017). Biorefinery of microalgal soluble proteins by sequential processing and membrane filtration. Bioresource Technology, 225, pp.151–158.
Schade, S.; Stangl, G.I.and Meier, T. (2020).Distinct microalgae species for food—Part 2: Comparative life cycle assessment of microalgae and fish for eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and protein. Journal of Applied Phycology, 32, pp.2997–3013.
Schilderman, P.A.E.L.; ten Vaarwerk, F.J.; Lutgerink, J.T.; Van der Wurff, A.; ten Hoor, F. and Kleinjans, J.C.S. (1995). Induction of oxidative DNA damage and early lesions in rat gastro-intestinal epithelium in relation to prostaglandin H synthese-mediated metabolism of butylated hydroxyanisole. Food and Chemical Toxicology, 33, pp.99-109.
Schwenzfeier, A.; Wierenga,P.A. and Gruppen,H.( 2011). “Isolation and Characterization of Soluble Protein from the Green Microalgae Tetraselmis Sp.” Bioresource Technology, 102 (19), pp. 9121–9127.
Schwenzfeier, A.; Helbig, A.; Wierenga, P.A.and Gruppen, H. (2012). Emulsion properties of algae soluble protein isolate from Tetraselmis sp. Food Hydrocoll. 30, pp.258–263.
Schwenzfeier, A.; Helbig, A.; Wierenga, P. A. and Gruppen, H. (2013). “Emulsion Properties of Algae Soluble Protein Isolate from Tetraselmis Sp.” Food Hydrocolloids 30 (1), pp. 258–263.
Seyfabadi, J.; Ramezanpour, Z. and Khoeyi, Z. A. (2011). Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. Journal of Applied Phycology, 23, pp.721–726.
Siddiki, S. Y. A.; Mofijur, M.; Kumar, P. S.; Ahmed, S. F.; Inayat, A.; Kusumo, F.; … and Mahlia, T. M. I. (2022). Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel, 307, 121782.
Soto-Sierra, L.; Stoykova, P. and Nikolov, Z. L. (2018). Extraction and fractionation of microalgae-based protein products. Algal Research, 36, pp.175–192.
Spolaore, P.; Joannis-Cassan,C.; Duran,E. and Isambert,A. (2006). “Commercial Applications of Microalgae.” Journal of Bioscience and Bioengineering 101 (2), pp. 87–96.
Stamer, A. (2015). “Insect Proteins-A New Source for Animal Feed.” EMBO Reports 16 (6), pp. 676–680.
Suzuki, T.; Nakai, K.; Yoshie, Y.; Shirai, T.; Hirano, T. (1993). Digestibility of dietary fiber in brown Alga, Kombu, by rats. Bull. Jpn. Soc. Sci. Fish, 59, 879–884. [CrossRef]
Teuling, E.; Wierenga, P.A.; Schrama, J.W.and Gruppen, H., (2017). Comparison of Protein Extracts from Various Unicellular Green Sources. Journal Agric. Food Chem. 65, pp. 7989–8002.
Till, B.E.; Huntington, J.A.; Early, W. P. R; Taylor-Pickard, J. and. Sinclair L.A.(2019). “Influence of Rate of Inclusion of Microalgae on the Sensory Characteristics and Fatty Acid Composition of Cheese and Performance of Dairy Cows.” Journal of Dairy Science, September. American Dairy Science Association.
Urbano, M.G.and Goñi, I. (2002). Bioavailability of nutrients in rats fed on edible seaweeds, nori (Porphyra tenera) and wakame (Undaria pinnatifida), as a source of dietary fibre. Food Chem., 76, pp.281–286.
Van den Berg, H; Faulks, R.; Granado, H.F.; Hirschberg, J.; Olmedilla, B.; Sandmann, G.; Southon, S. and Stahl, W. (2000). The potential for the improvement of carotenoid levels in foods and the likely systemic effects. Journal of the Science of Food and Agriculture, 80, pp.880-912.
Vernèsa, L.; Abert-Viana, M.; El Maâtaouib, M.; Tao, Y.; Bornard, I. and Chemat, F. (2019) .Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem 54, pp.48–60.
Vonshak, A.; Chanawongse, L.; Bunnag, B. and Tanticharoen, M. (1996). Light acclimation and photoinhibition in three Spirulina platensis (Cyanobacteria) isolates. Journal of Applied Phycology, 8, pp. 35–40.
Waghmare, A. G.; Salve, M. K.; LeBlanc, J. G. and Arya, S. S. (2016). Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresources and Bioprocessing, 3.
Wells, M. L.; Potin,P.; Craigie,J.S.; Raven,J.A.; Merchant,S.S.; Helliwell,K.E.; Smith,A.G. Camire,M.E. and. Brawley,S.H. (2017). “Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding.” Journal of Applied Phycology, 29 (2), pp. 949–982.
WHO, J. (2007). Protein and Amino Acid Requirements in Human Nutrition. World Health Organization technical report series, (935), 1.
Wong, K.; Cheung, P.C. (2001). Influence of drying treatment on three Sargassum species. Journal of Applied Phycology, 13, pp.43–50.
Wu, T.; Li, L.; Jiang, X.; Yang, Y.; Song, Y.; Chen, L.; … and Gu, Y. (2019). Sequencing and comparative analysis of three Chlorella genomes provide insights into strain-specific adaptation to wastewater. Scientific reports, 9(1), pp.1-12.
Young, V.R.; Pellett, P.L.(1994). Plant proteins in relation to human protein and amino acid nutrition. The American journal of clinical nutrition, 59(5), 1203S–1212S.
Zhang, D.; Liu, S.; Zhang, J. ; Zhang, J. K.; Hu, C. and Liu, Y. (2016). “In Vivo Effects of Aphanizomenon Flos-Aquae DC-1 Aphantoxins on Gas Exchange and Ion Equilibrium in the Zebrafish Gill.” Aquatic Toxicology, 177, pp.484–493.
Zheng, J.X.; Yin, H.; Shen, C.C.; Zhang, L.; Ren, D.F.and Lu, J.( 2020). Functional and structural properties of Spirulina phycocyanin modified by ultra-high-pressure composite glycation. Food Chem. 306, 125615.
Zhu, H. and Damodaran, S.( 1994). Heat-induced conformational changes in whey protein isolate and its relation to foaming properties. Journal of Agricultural and Food Chemistry, 42(4), pp.846-855.
