THE NUTRITIONAL VALUE OF GERMINATED SORGHUM
Sheren Fadhal Abbas1*, Faleeha Hasan Hussein1, Alia Zyara Hashim1
Department of Food Science, College of Agriculture, University of Basrah, Basrah, Iraq
* Corresponding author. Email: sheren.abbas@uobasrah.edu.iq
HNSJ, 2024, 5(6); https://doi.org/10.53796/hnsj56/16
Published at 01/06/2024 Accepted at 18/05/2024
Abstract
Sorghum [Sorghum biocolor (L) Moench] is an important cereal crop grown in diverse environments and belongs to the grass family. It is used as a staple food in developing countries, and its stalks and leaves are used for livestock feed. Sorghum grains also produce flour to prepare breads, 30%- and 70%-spelled pastries, porridges, and snacks, as well as cooking oil, alcohol, starch, and glucose.It contains a high percentage of protein, and Sorghum protein is characterized by its absence of colotinin, which is used by people with diabetes. Its grains are a rich source of the vitamin B group and phenolic compounds that are beneficial to health. Sorghumgrains are also included in As a supplement in concentrated feed for poultry due to its high protein content, reaching 12%. Protein is an important component that reflects the quality of Sorghum grains. Applying the seed germination process is a valuable technique in improving the nutritional contents of these grains, which are widely used in the world. This technology has proven effective in enhancing the nutritional value of these grains, especially in countries that depend on grains in their diet, as they usually stimulate a group of enzymes upon germination. These include amylase, protease, lipoxygenase, polyphenol oxidase, and peroxidase. These enzymes convert complex compounds into simple parts, which initiate complex physiological and biochemical changes in the seeds. Many bioactive compounds are formed that have proven many positive effects on human health.
Key Words: Sorghum, Germination, Chemical composition, Nutritional value
عنوان البحث
القيمة الغذائية للذرة البيضاء المنبتة
شيرين فاضل عباس1*، فليحه حسن حسين1 ، عالية زيارة هاشم1
1 قسم علوم الاغذية/ كلية الزراعة/ جامعة البصرة- البصرة/ عراق
*المراسلة الى: شيرين فاضل عباس ، قسم علوم الاغذية، كلية الزراعة، جامعة البصرة، البصرة، العراق
بريد الكتروني: : sheren.abbas@uobasrah.edu.iq
HNSJ, 2024, 5(6); https://doi.org/10.53796/hnsj56/16
تاريخ النشر: 01/06/2024م تاريخ القبول: 18/05/2024م
المستخلص
الذرة الرفيعة (الذرة البيضاء ذات اللونين) [Sorghum bicolor (L) Moench] هي محصول حبوب مهم يزرع في بيئات متنوعة وينتمي إلى العائلة العشبية. وهو خامس أهم محصول الحبوب في العالم. تُستعمل حبوب الذرة الرفيعة كغذاء أساسي في البلدان النامية، بينما تُستعمل السيقان والأوراق كعلف للماشية. تتم معالجة حبوب الذرة الرفيعة وتحويلها إلى دقيق لتحضير الخبز الخبز والمعجنات بنسبة 30% و 70% من الحنطة والعصيدة والوجبات الخفيفة. كما يمكن الحصول على زيت الطهي من حبوبها وصناعة الكحول وانتاج النشأ والكلوكوز، وتحتوي على نسبة عالية من البروتين ويتميز بروتين الذرة البيضاء بخلوه من الكلوتينين الذي يستعمل للمصابين بمرض السكري، وتعد حبوبه مصدراً غنياً لمجموعة فيتامين B والمركبات الفينولية المفيدة للصحة ، كما تدخل حبوب الذرة البيضاء كمادة مكملة في العليقة المركزة للدواجن لارتفاع نسبة البروتين فيها، إذ يصل 12%. يعد البروتين من المكونات المهمة التي تعكس نوعية حبوب الذرة البيضاء. شهدت الإنزيمات تطوراً كبيراً في مجال الصناعات الغذائية كالغذاء والمكملات الصناعية نظراً لانتشارها الواسع في العديد من العمليات الغذائية. يعد تطبيق عملية إنبات البذور تقنية قيمة في تحسين المحتويات الغذائية لهذه الحبوب التي يتم استعمالها بشكل واسع في العالم. أثبتت هذه التقنية فعاليتها في تعزيز القيمة الغذائية لهذه الحبوب خاصة في البلدان التي تعتمد على الحبوب في نظامًها الغذائي عادة ما تحفز مجموعة من الإنزيمات عند الإنبات. منها إنزيم الأميليز وإنزيم البروتيز و ليبوكسيجيناز وإنزيم البولي فينول أوكسيديز والبيروكسيديز اذ تقوم هذه الإنزيمات بتحويل المركبات المعقدة إلى أجزاء بسيطة والتي تبدأ التغيرات الفسيولوجية والكيميائية الحيوية المعقدة في البذور وتتكون العديد من المركبات الفعالة حيويا التي اثبتت العديد من التاثيرات الايجابية على صحة الإنسان.
الكلمات المفتاحية: الذرة البيضاء، الانبات ،التركيب الكيميائي، القيمة الغذائية
Introduction:
Grain crops it is one of the main basic sources in most parts of the world because of its essential role in the economic sector due to the shortage of these crops in some countries due to drought and salinity to which agricultural lands are exposed and different environmental conditions. They are annual and herbaceous plants and are classified as winter crops such as wheat, barley and oats, and summer crops such as Sorghum and yellow corn (Johansson et al., 2021).
Sorghum (high quality) is an industrially important fodder grain crop that ranks fifth in terms of importance and cultivated area. It is cultivated in Iraq in Wasit, Dhi Qar and Maysan, which tolerates high temperatures and drought (Al Hadi, 2019). Enzymes have experienced significant growth in the food industry for use as nutritional and industrial additives because they are widely used in many food processes. Enzymes are sourced from plans, animals and microorganisms during production and extraction (Saini et al ., 2017; Gupta et al., 2021). Enzymes, in general, have been introduced in the areas of food processing in abundance, especially amylase enzymes, because because of their importance in improving the quality of baked goods, and they are healthy and have no harm to human health (Missarah et al ., 2020 ; Matpan Bekler et al ., 2021). The alpha-amylase enzyme, an ancient human enzyme, is crucial in the food and pharmaceutical industries for starch analysis, bread production, and baby food production (Trabelsi et al., 2019; Desai et al., 2021) Amylase enzymes are classified from the group of hydrolysis enzymes.Germination is the process of grain absorbing water to allow embryo growth, resulting in swollen cells and a softer, more permeable cytoplasm (Hassan et al., 2020).
The importance of cereal crops
Pills play a crucial role in the lives of many people, especially in developing countries, as grains and their derivatives provide essential nutrients like carbohydrates, proteins, fibers, vitamins, and minerals. Due to their small size and low humidity, they are easily transported and stored (Stagnari et al., 2017; Sohail et al., 2021). Cereal crops like barley, wheat, millet, sorghum, maize, oats, and other crops belong to the Gramineae or Poaceae family (Layek et al., 2018). Nutrition scientists agree that excessive reliance on grain products for protein and calorie processing negatively impacts individuals’ nutritional and subsistence levels if not supplemented by animal-based food.
Biological Activities of Grains
Because grains are consumed on a global scale and are a staple diet in many nations, their consumption may have an effect on health outcomes. Grains include a significant amount of carbs, a useful source of energy, as well as other beneficial components, phytochemicals (Okarter and Liu, 2010), hence adding these grains to diets as whole grains or in the form of other products results in significant nutrtional differences. When comparing the nutritional profiles of whole grains with milled grains, dietary fiber, minerals, vitamins, and other bioactive components, such as polyphenol, which are antioxidants, are the most notable differences (Jonnalagadda et al., 2011). Whole grain varieties vary in their nutritional makeup and, as a result, in their bioactive chemicals and activities, which cause a range of biological effects in the body (Donkor et al., 2012). Different physiological reactions are associated with a variety of bioactive chemicals found in grains along with other constituents (Björck et al., 2012; Fardet, 2010). The functional properties of whole grains have been studied by numerous researchers in the past, but they have not yet been fully elucidated (Bjorck et al., 2012) .Dried rye flour and brown rice are not reliable sources of dietary fiber, as they have different amounts and compositions, compositions,despite their potential health benefits .Dried rye flour and brown rice are not reliable sources of dietary fiber, as they have different amounts and compositions, despite their potential health benefits (Food,US and Drug Administions [USFDA],2012) 23.8G/100. There are many explanations for how whole grains work , such as starch structure, solubility characteristics, and viscosity caused by fiber, combined with particle size ( Jonnalagadda et al .,2011). Food structure, and delatinization (Bjorck et al .,2012). Whole grains, rich in soluble fiber, short-chain fatty acids, minerals, and antioxidants, can help lower blood cholesterol levels, prevent diabetes, and prevent cholesterol production (Jonnalagadda et al., 2011). Polyphenolic antioxidants may also lessen oxidative stress and inflammation (Mateo Anson et al., 2010). Importantly, it was discovered that eating whole grains had better health benefits than eating milled grains; epidemiological studies supported this finding (Masters et al., 2010). Metabolic rate at rest, consuming whole grains reduced LDL and total cholesterol (Newby et al., 2007). Whole grain consumption is linked to a lower risk of diseases, but countries like Canada, the USA, and Australia don’t follow recommendations, with consumption only increasing by 20% in industrialized nations.
Australia and Canada have seen a decrease in whole grain consumption, with less than one-third of the recommended servings consumed of whole grains were ingested (Go Grains Health and Nutrition [GGHN], 2010). In Canada, 21%-66% of 19-year-olds do not consume the recommended amount. Barriers include ignorance, processed foods, and availability of other goods. Anti-nutritional substances in grains, like phytic acid, may limit the absorption of minerals like iron and zinc (Garriguet, 2007; Gupta et al., 2015).
“The European HEALTHIGRAIN” has acknowledged a unique grain processing technique that will enhance the nutritional profile and flavor of whole grains and, in turn, benefit consumer health (Delcour et al., 2012). Several anti-nutritional elements that are present in grains, such as phytic acid, may limit the availability of a number of minerals, including iron and zinc. restrict the absorption of these minerals (Gupta et al., 2015). A special grain processing method that will improve the nutritional profile and flavor of whole grains and, in turn, increase consumer health has been recognized by “The European HEALTHIGRAIN” (Delcour et al., 2012.(
Sorghum bicolor (L.) Moench is the fifth most productive grain globally, affecting white bread production by 30% to 70%. Its high protein content of up to 12% in chicken concentrated feed reflects its quality and benefits from growth elements, particularly in poultry concentrated diets (Tobias et al., 2018; Hassouni, 2019; Assefa et al ., 2020), the origin of maize, the belief is that it was first d developed in eastern and central Africa, with the exact date of human cultivation being unknown. However, it is widely accepted that it was being used in Egypt around five thousand years ago and in Assyria 700 years ago B.C.E.(Girma et al., 2020) Sorghum, a crucial field crop, is grown globally except in northwestern Europe due to low temperatures. Its grains produce cooking oil, alcohol, starch, glucose, protein, and a vitamin B group (Buschmann, 2018; Mardian et al ., 2020; Teressa et al ., 2021).




Figure 1. Grain Sorghum
Varieties of Sorghum
- Grain Sorghum bicolor: Its varieties are characterized by the production of abundant grain yield, and its grains are large in size compared to other types, and their color is white or yellow.
- Sugar corn Sorghum bicolor moench: The plants plants are long and contain sweet juice. The grains vary in color from red to brown or white.
- Fodder Sorghum: It includes the Sudanese hashish, which is distinguished by its thin stems with a length of 100-150 cm, branches and open branching clusters.
- Broom corn: It is distinguished by its thich stems, anxious leaves, and a short cluster stand.
Sorghum, which is grown for the purpose of producing seeds, contains several types, including Kafir, local, Hegari, Milo, Inqaz, and Brief Rabeh (Buschmann, 2018; Nyoni et al., 2020).
Chemical composition of Sorghum Varieties
Sorghumis one of the important crops in the world due to its ability to withstand drought and high temperatures, and the Sorghum grain consists of the main ingredients carbohydrates (65-63)% and protein (9.2-13.5%). Sugar and fat (2.9-3.5) %, ash (0.89-1.99) %, and fiber (1.22-2.89) % of the grain weight (Katami, 2004). The chemical composition of Sorghum varieties differs (Table 1) depending on genetic factors and the quantity and quality of protein (Hassouni, 2019). White sorghum, a global staple crop, is rich in nutrients and phenolic compounds, with three main parts: the bran layer, endosperm, and seed, making it a diverse and abundant cereal (Xiong et al., 2019).
Table 1. Chemical composition of Sorghum varieties
| chemical composition | Sorghum variety | |||
| kaffir | rescue | winner | Giza | |
| Moisture | 14.00 | 14.20 | 10.00 | 11.00 |
| Ash | 0.920 | 1.500 | 1.0 | 1.300 |
| Oil | 2.20 | 2.00 | 3.30 | 3.50 |
| protein | 13.00 | 11.20 | 8.70 | 6.20 |
| fiber | 1.800 | 1.500 | 2 | 2 |
| carbohydrates | 68.08 | 69.6 | 75 | 76 |
Reference: (Abbas, 2022)
Fields of the grain industry
Cereals are used in various industries, including bread, pastries, sweets, biscuits, baby food, cake, and rice. They also produce cellulose, glues, adhesives, oils, chemicals, alcohols, cosmetics, insulation materials, alcoholic beverages, and milk substitutes. Cereals also contribute to the food industry by providing improved products and milk substitutes (Zhao et al ., 2019).
Malt
Malt is the specific germination of grain or legume seeds under controlled conditions, primarily associated with barley. It is widely used in the food industry and is produced using the beta-amylase enzyme from ungerminated barley grains (Al-Joufi, 1988; Baranwal, 2017), Malt was also produced from white corn.
Malt germinates grains by soaking them in water, causing the development of enzymes to modify starches into sugars like glucose, maltose, and maltodextrines, and proteases to break down proteins (Mäkinen and Arendt, 2015). Seeds stored for years experience weakness and decreased germination, while increased storage periods decrease enzymatic activity, while gibberellic acid activation enhances enzyme activity and germination characteristics (Ansari et al., 2013). The aim of stimulating seeds is to increase the average rate of germination, reduce the time required for germination, and improve seedling growth in suitable and unsuitable conditions.
Germination processing
According to Hefni and Witthöft (2011), germination is a natural processing method used to biologically activate grains and enhance their nutritional and functional qualities (Figure 2) . Germination of cereals and legumes (288 seeds) requires optimal humidity levels, oxygen accessibility, and suitable temperatures for optimal metabolic processes, affecting the bioactive compounds produced during sprouting (Sangronis and Machado, 2007). Preharvest sprouting (PHS) is a field-based method that adjusts environmental parameters like moisture, temperature, and humidity to produce essential nutrients like soluble dietary fiber, vitamins, minerals, antioxidant, and phytochemicals (Van Hung et al., 2011).
To boots the nutrititional value of grains,germination must be conducted for a number of days(Rakcejeva et al .,2014). Spruces, a rich source of nutrients like glucosinolates, phenolics, and selenium,are essential for health. They alos contain phytochemicals, vitamins, minerals, enzymes, and amino acids which are beneficial for overall health (Gan et al .,2017) The The biological singnificance of nutritions sprouts has recently received increased attention from researchers (Maton et al.,2010 ). The study found a decrease in anti-nutritional substancs like trypsin inhibtitors and phytic acide , while an increase in health –improving substances like glucosinolates showed anti-cancer activity.
As a result, sprouting can boost nutritional levels ,which benefits human health and lowers the risk of a number of major diseases(Sangronis and Machado.,2007). Although the precise nutritional makeup of sprouts is unclear since a variety of legumes have different nutritional profiles, germination fundamentally altered the nutritional and physicochemical properties of seeds to support the growth of young plants(Noda et al.,2004). Germinated grains improve human health by containing beneficial nutrients and reducing antinutritional elements. Germination, a process where grains are briefly soaked in water, enhances their technical and nutritional qualities (Kaukovirta-Norja et al., 2004). Kernels’ germination conditions and water content significantly influence grain metabolism, leading to the synthesis and release of health-maintaining metabolic chemicals during embryonic stages (Hübner and Arendt, 2013).

Figure 2. Germination of sorghum
Mechanism of germination
Germination involves sterilization, steeping, and sprouting in a controlled environment. To reduce microbial load, seeds are sterilized with water, with sodium hypochlorite solutions being the most effective. 0.07% NaCIO solution is commonly used (Limón et al., 2014). Sterilization is done at room temperature for 15–30 minutes using a solution with 85 g of seed weight per solution volume, pure ethanol, or 70% ethanol, and a 0.2% formaldehyde solution for 3- minutes (Nour et al., 2015; Pajk et al., 2014). Results of a few studies demonstrate how sterilization affects seed germination. While sterilizing is rarely used in research (Guo et al., 2012; Guajardo-Flores et al., 2013), Seed sterilization is crucial for germination, involving rehydration in water at a 1:1.5 to 1:20 ratio, with a germination time of up to 24 hours (Gan et al., 2017). Dry grains’ metabolic activity increases during soaking, requiring careful consideration of light, temperature, humidity, watering, and time for effective seed germination. Ideally, a 20–30 °C temperature, daily watering, and periodic water changes are crucial for successful growth (Kandil et al., 2015). Water must be present for germination to begin, which starts a protracted biochemical and physiological process that supports the developing baby plant and ends when the embryonic axis lengthens. Radiation forming around the grain’s embryo is the clear indication that the germination process was finished (Bewley and Black, 1994). According to Nelson et al. (2013), Water penetration enhaces seed rehydration, stimulates gibberellin synthesis, and induces hydrolytic enzyme gene expression. Optimal humidity and temperature stimulate hormones in grains, releasing nutrients from the germ, endosperm, and scutellum and promoting debranching and hydrolytic enzymes (Gan et al., 2017). The time needed for sprouting varies from seed to seed and is typically between three and five days for edible beans, for instance. The germination method is easy, inexpensive, environmentally friendly, and suggested as a safe way to sprout a seed quickly (Gan et al., 2017). Gibberellin, a hormone released from embryos, stimulates enzyme release and reduces inhibitor activity in seeds during germination, transforming them from dormant to active metabolism (Iordan et al., 2013). Enzymes break down stored proteins and carbohydrates into smaller molecules for the developing plant to thrive, as most chemicals are not soluble in water (Miransari and Smith, 2014). Germination in grains undergoes numerous phytochemical and physiological changes, leading to a complex nutritional flow and breaking down complex substances into smaller molecules for plant growth and photosynthesis (Theodoulou and Eastmond, 2012). Numerous nutrients and other bioactive substances increased throughout the germination process (Donkor et al., 2012) and decreased when these substances were used by the developing plant (Yang et al., 2001). According to Hung et al. (2012), sprouted waxy wheat has a better and more advantageous nutritional profile than nonsprouted wheat because it has higher levels of phenolics, free amino acids, and dietary fiber. Another study revealed that following 102 hours of germination at 20-25 ºC, grains have 2-3 times more minerals and a 2-fold higher concentration of α-tocopherol (Ozturk et al., 2012).
The level of folate was found to be 3.6 times higher in the same study (Koehler et al., 2007; Yang et al., 2001). The study found that ascorbic acid, tocopherol, and beta-carotene were present in higher amounts in ungerminated wheat grains, increasing steadily after germination. Vitamin C, alpha-tocopherol, and beta-carotene values reached optimal levels after 7 days, while ferulic and vanillic acid levels also increased.The results of germination experiments can vary due to various factors like grain types, germination conditions, and laboratory technology, with sugar production varying between the ungerminated and control groups (Moongngarm and Saetung, 2010). Similar findings were made in another study, where the antioxidant capacity of s sprouted rice showed both an increase and a decrease. Scavenging activity of the rice variety was used to determine the antioxidant activity (Imam et al., 2012). Comparing findings from other investigations requires caution due to varying ideal conditions based on grain type, parameter, analytical techniques, temperature, time, moisture, oxygen availability, whole grain type, and environmental conditions (Mak et al., 2009). The ideal conditions for cereal grains vary based on grain type, research parameters, and analytical techniques, with germination and development phases separated by dormancy. Seeds serve as reproductive units during germination, ensuring plant species’ survival. Wheat grain consists of endosperm, bran layer, embryos, and germ. Enzymes break down complex components like proteins, lipids, and carbohydrates into simple molecules, typically stimulated during germination.
These enzymes are primarily found in the germ, bran, and higher aleurone layer (Poutanen, 1997). Amylase, protease, polyphenol oxidase, peroxidase, and lipoxygenase are essential enzymes in plants for breaking down complex starch, proteins, and fats (Rani et al., 2011). These enzymes break down complicated substances into simpler components, which start the seeds’ intricate biochemical and physiological transformations.
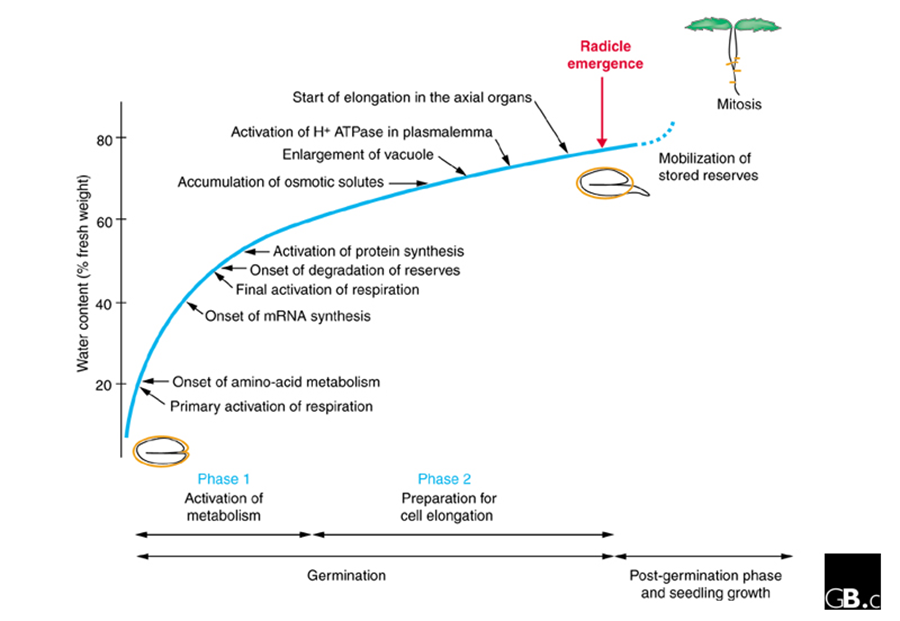
Figure 3. Germination of seed and physiological process (Bove et al., 2001)
Bioactive compounds
The plants produce a huge array of organic substances, none of which directly contributed to the growth and development of the plants. These substances, which are known as secondary metabolites, can be found in different concentrations in different taxonomic groups of plants. As opposed to primary metabolites, which are created by all types of plants and play a major role in the development and growth of the plant, secondary metabolites plants also generate primary metabolites including a acyl acids, phytosterols, organic acids, and amino acids (Hussain et al., 2012). secondary metabolites, which plants can only create in limited quantities, have complex structures and biochemical processes. Traditionally, researchers haven’t given these molecules much thought because they’re thought to be biologically inconsequential (Parsaeimehr et al., 2011).Contrary to biochemists, pharmaceutical researchers gave secondary metabolites (organic substances) a lot of thought and began aggressively researching their biochemical properties in the 1850s. A number of substances, including dyes, polymers, fibers, glues, oils, waxes, flavoring agents, perfumes, and medications, were produced from the study of these bioactive molecules, which is an interesting development both on the academic and industrial levels. Due to the significant biochemical features of these molecules, researchers have concentrated on creating novel drugs, herbicides, insecticides, and antibiotics (Grindberg et al., 2011). These molecules are categorized into three main groups (terpenes, phenolics, and compounds containing nitrogen) based on their synthesis processes (Krzyzanowska et al., 2010). The mevalonic pathway, also known as the shikimic acid pathway or the mevalonic pathway, has been studied in relation to the synthesis of terpenes, phenolic compounds, and secondary metabolites that contain nitrogen. The tricarboxylic acid pathway has also been studied.Many different drugs are made in the pharmaceutical business employing these secondary metabolites (Parsaeimehr et al., 2011).
Phenolic Compounds
Numerous studies have identified polyphenolic compounds (Table 2) as one of the main antioxidant elements in whole grains (Zieli’nski and Kozowska, 2000). Due to their inherent antioxidant properties, phenolic substances stabilize radical molecules created during oxidation. Vitamins, sterols, phenolics, and phytic acid are a few examples of the many antioxidant components found in whole grains. The aforementioned substances are primarily present in the bran or germ of whole grains (Fardet et al., 2008). All of the aforementioned bioactive chemicals have some level of antioxidant activities, and the germination process also has an impact on them. The antioxidant impact of these substances was demonstrated by numerous in vitro investigations, but the in vivo antioxidant effect has not yet been fully understood (Fardet et al., 2008). It’s intriguing to learn that while some bioactive substances exhibit antioxidant properties in vitro, it’s possible to miss these substances in vivo. Plants can have polyphenols in soluble or bound forms, although the majority of edible grains have a high ratio of bound phenolics (Agati et al., 2012). Shikimate-phenylpropanoids flavonoids pathways are used by plants to produce polyphenolic contents (secondary metabolites), which are composed of a number of structurally and functionally varied substances (Krzyzanowska et al., 2010). According to Parsaeimehr et al. (2011), the majority of phenolic compounds have estrogenic properties. These compounds are classified as hving one or more aromatic rings and hydroxyl substituents, as well as various functional derivatives such esters, methyl ethers, glycosides, etc. According to Krizanowska et al. (2010), polyphenolic compounds can be separated into two groups: soluble phenols, such as phenolic acids, flavonoids, and quinones, and insoluble phenols, such as condensed tanins, lignins, and cell wall-bound hydroxycinnamic acids. Polyphenolic compounds contain a wide variety of substances, including tannins, lignin, flavonoids, isoflavonoids, and furanocoumarins. The enormous and diverse units of polyphenolic chemicals that are present in plants make up another family of phenols called flavonoids. There are two significant groups of flavonoids: flavones, which include apigenin, luteolin, and chrysoeriol, and flavonols, which include quercetin, kaempferol, and isorhamnetin. Gallic and caffeic acids, vanillic acid, cinnamic acid, and coumaric acid are all members of the phenolic acids class (Krzyzanowska et al., 2010). Typically, sprouted seeds include phenolic acids and flavonoids, which are recognized as anti-oxidants and may be used as useful components (Fu et al., 2011). These seeds may be the ideal substitute for fruits and vegetables in our healthy diets because sprouted seeds are a rich source of bioactive chemicals and phenolic compounds.
Table 2. Phenolic compounds in different germinated germinated cereals sprouts
| Grain type | Phenolic compounds | Analytical method | Remarks/ results |
References |
| Sorghum | Total polyphenol contentIndividual polyphenol content | UV spectrophotometer |
Increased | Donkor et al. (2012) |
| Maize | Phenolic contents | UV spectrophotometer |
Increased | Mihafu et al.(2017) |
| Barley | Total flavonoid content Total Phenolic content Antioxidant activity |
UV spectrophotometer |
Increased | Aborus et al. (2017) |
| Soybean | Total flavonoid content Total Phenolic content Antioxidant activity |
UV spectrophotometer |
Increased | Xue et al. (2016) |
| Buckwheat | Individual Phenolic | HPLC | Increased | Nam et al. (2015) |
| Wheat | Total Phenolic content Individual phenolic Antioxidant activity |
UV spectrophotometer |
Increased | Zilic et al. (2014) |
| Finger millet | Polyphenols content | UV spectrophotometer |
Increased | Sudha and Usha (2014) |
| Rice | Total polyphenol content Antioxidant activity |
UV spectrophotometer |
Increased | Imam et al.(2012) |
| Waxy wheat | Total Phenolic compounds Antioxidant activity |
UV spectrophotometer |
Increased | Hung et al.(2012) |
| Oat, | polyphenol content Antioxidant activity |
UV spectrophotometer |
Increased | Panfil et al.(2014) |
References: Hassan et al. (2020)
Germinated grains and polyphenols
It has been demonstrated via numerous investigations on cereal grains that the germination process can increase the amount of phenolic chemicals that can be extracted using solvents. According to some writers (Kim et al.,2013;Tang et al.,2014) the de novo synthesis and transformation can be blamed for the increase in water –soluble polyphenolic chemicals during the sprouting phase .A negative effect of seed germination was also noted because numerous studies revealed that sprouted seeds had less polyphenol content.This paradoxical outcome is linked to an increase in moisture content during the germination phase, when soluble phenols may be lost (Guo et al.,2012).These contradictory finding are mostly attributable to variation in soaking time ,temperature ,germination rates, and drying techniques for the germinated grains .Numerous in vitro investigation on cereal grains ,including wheat (Van Hung et al.,2011), sorghum (Donkor et al.,2012), buckwheat (Alvarez-Jubete et al.,2010), and rice (Imam et al.,2012), have reported increased phenolic contents and antioxidant activity. According to Demeke et al., (2001), increased polyphenol oxidase activity (PPO) may be the cause of the higher polyphenol levels in germinated grains compared to non-germination grains .The solubilization of condensed tannins during water soaking and its migration to the outer layer during may be the cause of the rise in polyphenols observed during seed germination. Due to increased activity of the endogenous enzymes phytase, which hydrolyzes phytic acid, phytate concentration decreased during maize germination (Pawar et al.,2006).Thedgree of phytate breakdown is influenced by a number of variables, including grain type, germination stage, pH, moisture content, temperature, phytate solubility ,and the presence of certain inhibitors (Egli et al.,2002). In a study by Liukkonen et al.,(2003), rye grains were germination for 6 days at various temperatures ,including 5,10, and 25 C. While all treatments showed an increase in methanol-extractable phenolic chemicals, the samples germinated at 25 ºC showed the largest increase. According to Wang et al. (2014), the synthesis or activation of a range of hydrolytic enzymes began in grains during germination, causing various structural changes or the creation of novel molecules with high bioactivity and nutritional value. In a different study, discovered that while the main soluble phenolic compounds in brown rice, 6-O-feruloylsucrose and 6-O-sinapoylsucrose, dropped after germination for 24 h, the free phenolic compounds, including ferulic acid and sinapinic acid, considerably increased. According to Shohag et al. (2012), the total phenolic content of soybean and mung bean seeds and sprouts reduced after germination compared to other seed contents because phenolics were diluted after imbibition and growth, and water absorption was higher. In contrast, when moisture content is removed when calculating on a dry weight basis, the total phenolics content increased with germination time (Cevallos-Casals and Cisneros-Zevallos, 2010). According to Imam et al. (2012), enhanced antioxidant activity in a variety of grains has generally been linked to total antioxidant activity. Only oats contain the phenolic chemicals known as avenanthramides. They are said to contribute to the flavorful freshness of oat products. After germination, it was noted that these chemicals increased by around 20% (Skoglund et al., 2008).
Tannins, phenolic substances with a high molecular weight, have historically had a negative nutritional impact because they may complex minerals and create insoluble complexes with proteins. On the plus side, they support antioxidant activity and the breakdown products themselves possess antioxidant qualities. According to studies on sorghum (Dicko et al., 2005) and millet (Hemalatha et al., 2007), tannins are partially broken down during germination.
Germination and viability of embryos
Germination has two definitions; the first is agricultural, which is the process of emergence of seedlings above the surface of the soil, so that it has the ability to form a new plant. As for the physiological definition of germination, it is a morphological and physiological change arising from the absorption of water by the seed so that it has the ability to tear the seed coat, the growth of the embryo, the formation and emergence of the root, which depends on the amount of stored food. It is necessary to decompose it and benefit from it during the growth process, as the seed germination process is one of the important things to increase the effectiveness and vitality of the embryos, as the dryness of the grain reduces the germination rates, and to increase the germination of the seeds, the moisture content of the germinated grains, the temperature, and the use of some chemical stimuli to accelerate growth and germination must be increased. Chemicals used for germination, gibberellic, acetic acid, ascorbic acid, and salicylic acid, which increase root growth. (Sghaier-Hammami et al., 2020). The germination process takes place at a temperature of 25 °C in distilled water, by soaking the seeds, then placing them on plates covered with different filter papers for germination, explained (Hassouni, 2019) the germination of seeds increases the percentage of glucose and proline, so the use of distilled water for germination leads to an increase in the percentage of germination from 52% to 59% and from 16% to 24% at the highest levels of salinity, and the chemical composition and size of the seed has an impact on the quality of germination (Šēnhofa et al., 2016; Ariska, 2020;).

Figure 4. Germination grain crops. Reference: (Abbas, 2022)
Factors affecting on germination
There are many factors that affect the process of seed germination during the life cycle of plants and their tolerance to the environmental conditions they are exposed to, such as temperature, light, drought, heavy metals, salinity, and lack of water, which have an impact on the formation of plants such as growth, shape, and reproduction (Abu-Saied et al., 2017; Daddario et al., 2017). Matsa et al. (2021) showed that light has an effect on the process of seed germination, as it has the ability to germinate in dark and light conditions, meaning that some of them are affected or not affected by light, and light has a relationship with the germination process, as some plants need light for their growth or germination, such as lettuce and carrot seeds that It is distributed and scattered on the ground without covering it with soil, due to the small size of the seeds and the lack of the stored nutrients that are sufficient for seed germination. ( El-Naim et al ., 2012 ), and among other factors that have an impact on seed germination is the oxygen that is important for the seed respiration process at the beginning of germination, as the respiration rate increases with the increase in the amount of water absorption, and the respiration rate varies according to the plant, growth periods, and the internal factors of the seed or grain from In terms of physiological maturity, chemical composition, environmental factors, and gases surrounding the seed, and that the absorbed respiration coefficient is the ratio between the volume of oxygen and carbon dioxide RQ = CO2 / O2 And this rate indicates the amount of materials that were consumed in the process of respiration of carbohydrates, proteins and fats ( Abu-Saied et al ., 2017 ).
Enzymes
Enzymes are defined as all the proteins that catalyze chemical reactions and convert the reaction material or substances into one or more of the reaction products, as the reaction materials are associated with the enzyme at sites called active sites, and the active site is often located in one of the largest cracks on the surface of the enzyme. They are also highly effective biological adjuvants (Saini et al ., 2017; Williams, 2019), as it is found in all living organisms and also works to accelerate basic biochemical reactions, and enzymes are highly specialized, and some do not need chemical groups to activate their work, and others need an additional chemical substance, which is called the cofactor, as it can the catalysts should be one or more organic ions or an organic mineral molecule, and the enzymes are characterized by accuracy, high efficiency, and work in moderate conditions, as well as their use in reducing residues, which made them suitable in many applications ( Rana and Paul., 2020 ).
Bioactive compounds in cereals
Multiple studies indicated the presence of many aromatic compounds in grains, most of which are due to active chemical groups, which are groups of alcohols, saturated and unsaturated hydrocarbons, ketones, aldehydes, terpenes, and esters in grain varieties (Xiong et al ., 2019 ). Cereals are the main sources of energy and protein in the diet. Sources contain a wide range of bioactive compounds, vitamins, fiber and minerals in fruits, vegetables and their derivatives. However, some of the bioactive compounds are unique in nature and only found in grains (Saini et al., 2019). Phenolic compounds are defined as secondary metabolic compounds in plants, characterized by their basic structure with the presence of one or more aromatic rings linked to several free hydroxyl groups or linked to other groups such as ester, ether, methyl, the difference in the number of rings and the number and type of groups associated with them makes them divided into several groups, the most important of which are acids Phenolics, flavonoids, and flavonoids represent the bulk. Phenolic compounds are found in many foods of plant origin, specifically in fruits, vegetables, drinks, red wine, tea, coffee, fruit juice, grains, oilseeds, and legumes. Phenols are classified according to the number of carbon atoms in the basic structure of them ( Mokhtarpour et al ., 2014), phenolic acids are the simplest phenolic acids present in all sorghum grains at a concentration of 445 to 2850 µg/ gram. Phenolic acids can be divided into two categories: hydroxybenzoic acid and cinnamic acid, and among the phenolic acids found in sorghum grains they are valic, protocateic- cinnamic, coumaric, hydroxybenzoic, ferulic, caffeic and sennapic acids. Phenolic acids are found in the endosperm, peel, and testa of grains, while flavonoids are natural phenolic compounds resulting from secondary metabolism. It means yellow in Latin, and it is the generic term for a large group of phenolic compounds that were first recognized by the world Albert Szent-györgyi classified it as a B vitamin It is found in high concentrations in the aerial part of the plant, and is found in most plant varieties, especially the higher ones, and is widespread in angiosperms, gymnosperms, and almost non-existent in algae, it is also found in mosses as well as in monocotyledonous plants (Zamora-Ros et al., 2016).
Follow-up of the development of the amino acids of Sorghum grains
The amino acid content of thirteen amino acids was detected using an Amino acid analyzer technique for the sorghum variety (Kaffir). The percentage of amino acids for the Kaffir variety was different according to Table (3)
Table 3. The percentage of amino acids of sorghum (Kaffir) at different germination periods
| .top no | detention time | The name of the acid | Unsprouted kefir 24 | Kaffir germination 96 hours |
| 1 | 3.78 | Aspartic acid | 20.4 | 44.5 |
| 2 | 4.18 | Glutamic acid | 31.1 | 51.4 |
| 3 | 4.92 | Asparagus | 14.8 | 38.9 |
| 4 | 7.50 | I will owe | 19.8 | 37.4 |
| 5 | 8.19 | Serein | 17.4 | 42.6 |
| 6 | 10.49 | Argentine | 13.6 | 38.6 |
| 7 | 14.77 | methionine | 17.6 | 36.8 |
| 8 | 15.37 | whining | 18.3 | 38.7 |
| 9 | 17.46 | valine | 20.6 | 41 |
| 10 | 18.58 | Broline | 14.3 | 39.2 |
| 11 | 22.36 | vinyalanin | 15.6 | 42.8 |
| 12 | 24.28 | Lysine | 14.9 | 43.2 |
| 13 | 27.36 | Tyrosine | 13 | 37.8 |
Reference: (Abbas, 2022)
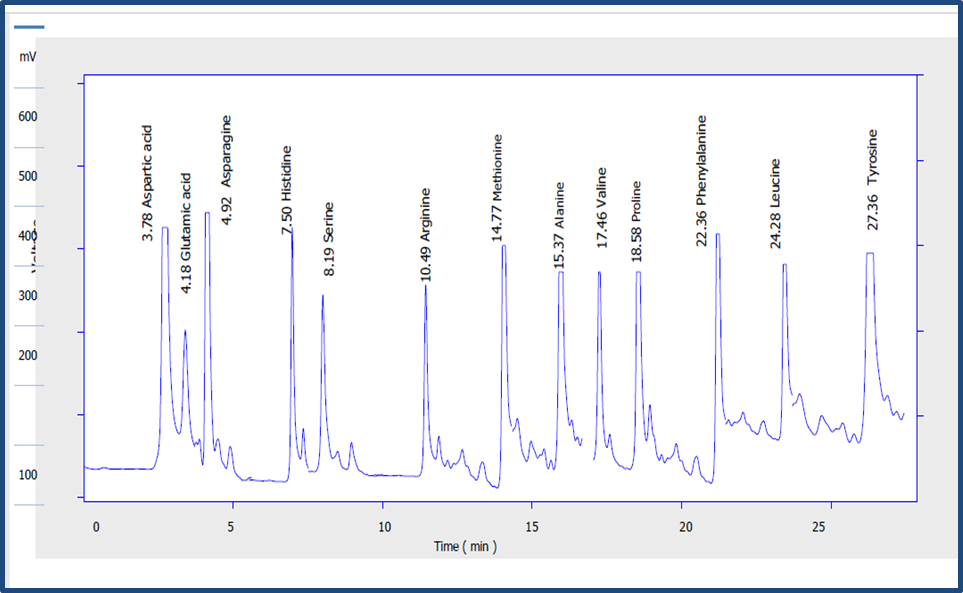
Figure 5. Characterstic amino acid profile of sorghum (kefir) Water- and fat-soluble vitamins by HPLC technology for Sorghum grains. Reference: (Abbas, 2022)
The fat-soluble vitamin analysis’s findings demonstrated an increase in each vitamin’s concentration as well as a change in the concentrations of these vitamins and the degree to which the device was sensitive to their presence during the germination phases. The table’s results demonstrated the superiority of vitamin K and vitamin E, two naturally occurring antioxidants found in grains, during the 96-hour germination phases of the kaffir type, while it was discovered that the Kaffir variety’s germination time of 96 hours was longer than that of unsprouted grains, the value in the table indicate a decrease in the content of the concentrations of fat-soluble vitamins in unsprouted grains. The concentrations can be followed through Table (4) and Chart (6) for fat-soluble vitamins Ojo et al. (2020).
Table 4. Concentration of fat-soluble vitamins: micro gram/ 100 g
| A | K | E | samples |
| 17.58 | 4.89 | 15.22 | Non-germinating kefir 24 hours |
| 22.58 | 8.49 | 20.15 | Kaffir germination 96 hours |
Reference: (Abbas, 2022)
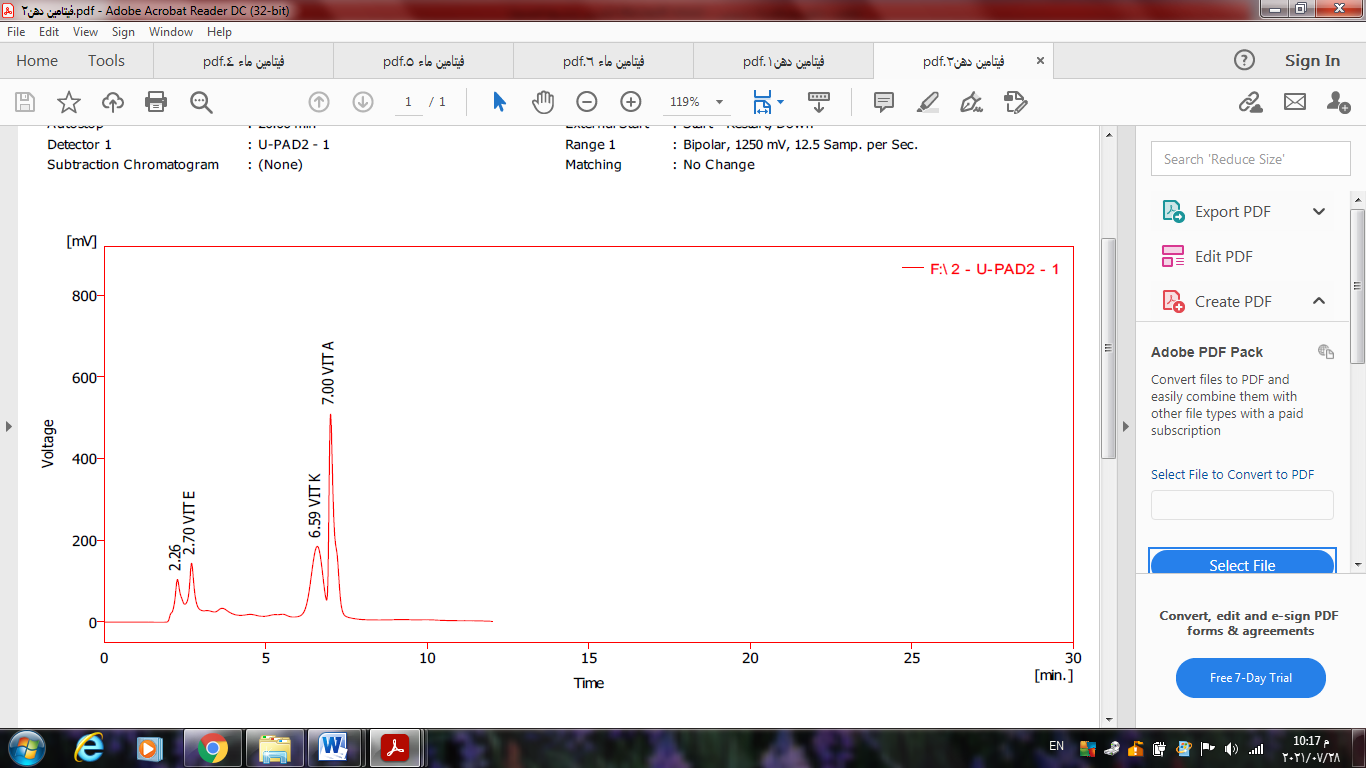
Figure 6. Chart for fat-soluble vitamins Reference: (Abbas, 2022)
Table 5. Concentrations of water-soluble vitamins in microgm/ 100g during germination periods.
| C | B9 | B3 | B2 | samples |
| 9.57 | 12.85 | 6.15 | 8.25 | 24 Kaffir germinating-Non hours |
| 12.44 | 16.87 | 9.14 | 10.26 | Kaffir germination hours96 |
Reference: (Abbas, 2022)
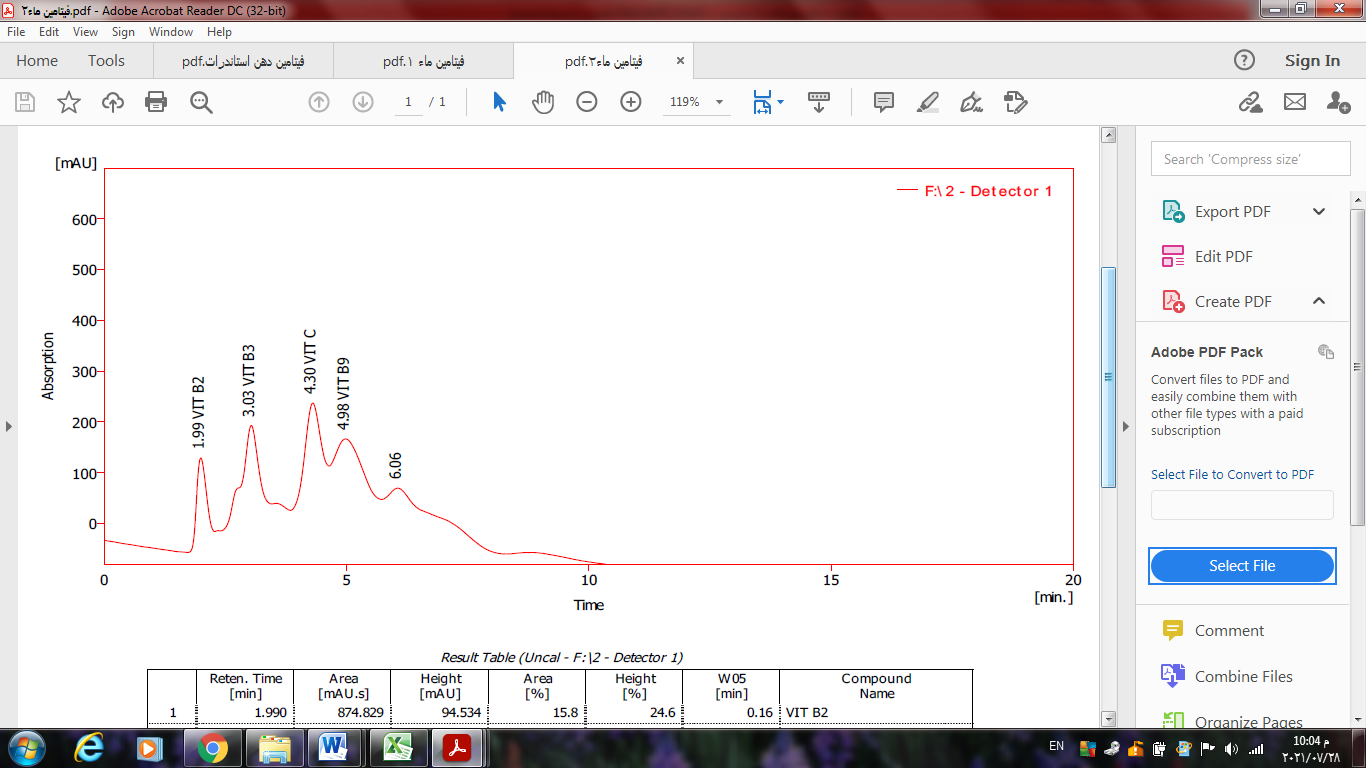
Figure 7. Chart for water -soluble vitamins. Reference: (Abbas, 2022)
Diagnosis of fatty acids in Sorghum
Fatty acids were identified using the gas chromatography technique connected to the mass spectrometer to diagnose the saturated and unsaturated fatty acids in the Kaffir cultivar before germination, as it was observed through the profile that there were 30 peaks belonging to the fatty acids, as the highest peak reached 2 for the saturated fatty acid Hexadecanoic acid methyl ester, whose common name is palmitic acid, with a percentage of 12.00 %. A saturated fatty acid at peak 7, with a percentage of 4.87%, belongs to the saturated fatty acid Octadecanoic acid, methyl ester, which is the stearic acid known as waxy acid, Oxiraneoctanoic acid, 3-octyl-methyl ester, Cis fatty acid methyl ester, and the presence of single fatty acids at peak 12 by 8.84%. 0.27% for the compound Methyl 18-methylnonadecanoate , and cyclic fatty acids at apex 20 with a percentage of 4.76% Cyclopropaneoctanoic acid, 2-[2-[(2-ethylcyclopropyl) methyl] cyclopropyl] methyl , and branched fatty acids at apex 14 with a percentage of 2.84% for compound 2,6 – Bis (3,4-methylenedioxyphenyl)-3,7-dioxabicyclo(3.3.0)octane , peak 15 of 0.68% for Bis (3,4-methylenedioxyphenyl) 3,7-dioxabicyclo 3.3.0 octane 2,6 , which is It is one of the biologically effective anti-inflammatory and anti-cancer compounds and an inhibitor of fungal growth (Hexadecanoic acid , methyl ester).
Table 6. Fatty acids saturated and unsaturated for non-germinated kefir variety
| Trade Name | Chemical name | R- time | Area% | top noPeak |
| Palmitic acid | Hexadecanoic acid, methyl ester | 15,172 | 12.00 | 2 |
| Stearic acid | Octadecanoic acid, methyl ester | 17,301 | 4.87 | 7 |
| Behenic acid | Docosanoic acid, methyl ester | 20,742 | 0.73 | 22 |
| turated fatty acidsUnsa | ||||
| Linoleic acid | 9,12- Octadecadienoic acid, methyl ester | 16,955 | 8.18 | 4 |
Reference: (Abbas, 2022)
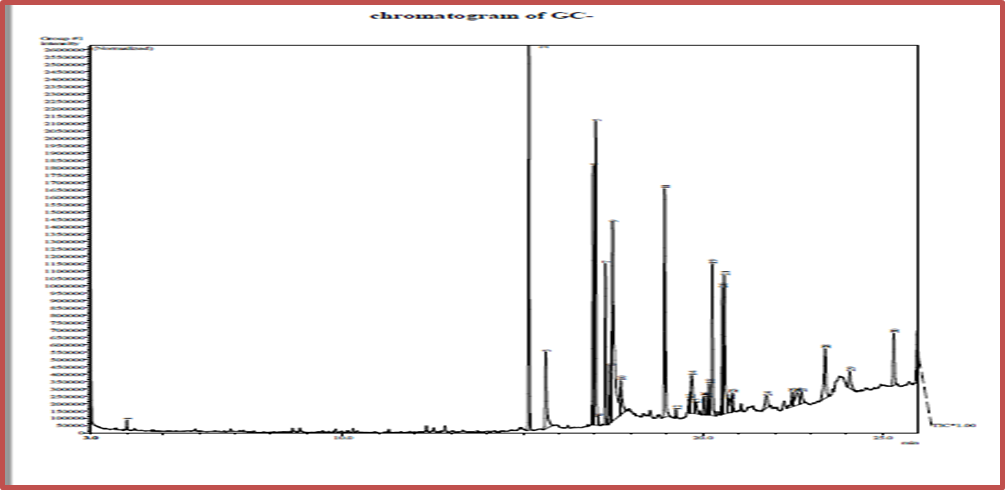
Figure 8. Gas chromatography-mass spectrometry of ungerminated sorghum, Kaffir cultivar. Reference: (Abbas, 2022)
Saturated and unsaturated fatty acids were diagnosed in white corn, Kaffir variety, with a period of 96 hours, germination, and the presence of 20 peaks, most of which belong to saturated and unsaturated fatty acids. The acid compositions differ by a similarity of 98%, as the highest peak 2 was 15.26% for saturated palmitic fatty acid. Hexadecanoic acid, methyl ester And at peak 4, with a rate of 31.46% for the compound 9,12- Octadecadienoic acid, methyl ester, and at peak 5, with a rate of 27.61% for the compound 13- Octadecenoic acid , methyl ester. fatty Single Hexadecenoic-7 acid, methyl ester , for peak 1, by 0.27% , acids by 0.27%, branched acids at peak 7, by 0.49% 9,12 – Octadecadienoic acid (Z,Z) ) and cyclic compounds at top 10 by 0.35% Cyclopropaneoctanoicacid,2-[2-[(2-ethylcyclopropyl) methyl cyclopropyl] methyl (Awonyemi et al., 2020) , and this is what the study showed that germination has an effect on the levels of acidity.Saturated and unsaturated fatty acids in the studied grains and their importance to the human body as essential acids.
References
- Aborus, N.E.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T.; Vulić, J. and Ilić, N. (2017). Powdered barley sprouts: composition, functionality and polyphenol digestibility. Int. J. Food Sci. Technol., 52(1):231–238.
- Abu-Saied, M. A.; Wycisk, R.; Abbassy, M. M.; Abd El-Naim, G.; El-Demerdash, F.; Youssef, M. E., … and Pintauro, P. N. (2017). Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions—Fabrication and sorption studies. Carbohydrate polymers, 165, 149-158.
- Agati, G.; Azzarello, E.; Pollastri, S. and Tattini, M. (2012). Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 196:67–76.
- Al-Hadi, M. Q. S. (2019). Effect of seed storage priming with different storage periods on seed vigor, growth and yield of Sorghum bicolor L. Ms. c Thesis, College of Agriculture, University of Karbala, Department of Field Crops, pp. 1-149.
- Al-Jawfi, F. K. A. (1988). Evaluation of commercial malt and fine grain malt of barley by using its extracts in the pharmaceutical industries. Ms. C Thesis, College of Agriculture, University of Baghdad.
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K. and Gallagher, E. (2010). Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 119(2):770–778.
- Ansari, O.; Azadi, M.S.; Sharif-Zadeh, F. and Younesi, E. (2013). Effect of hormone priming on germination characteristics and enzyme activity of mountain rye (Secale montanum ) seeds under drought stress conditions. Journal of Stress Physiology and Biochemistry, 9(3):61-71.
- Ariska, N. (2020). Viability Test and Breeding Growth on Poly-embryos Seeds. In IOP Conference Series: Earth and Environmental Sci., 515(1): 012067.0. IOP Publishing.
- Assefa, A.; Bezabih, A.G.; irmay, G.; Alemayehu, T. and Lakew, A. (2020). Evaluation of sorghum (Sorghum bicolor) L. Moench variety performance in the lowlands area of wag lasta, northeastern Ethiopia. Cogent Food and Agriculture, 6(1):1778603.
- Awonyemi, I.; Abegunde, M.S. and Olabiran, T.E. (2020). Analysis of bioactive compounds from Raphia taedigera using gas chromatography–mass spectrometry. Eurasian Chemical Communications, 2(8): 938-944.
- Baranwal, D. (2017). Malting: an indigenous technology used to improve the nutritional quality of grains: a review. Asian J. Dairy Food Res, 36: 179-183.
- Bewley, J.D. and Black, M. (1994). Seeds. In: Seeds. Springer, Boston, pp. 1–33.
- Björck, I.; Östman, E.; Kristensen, M.; Anson, N.M.; Price, R.K.; Haenen, G.R. and Welch, R.W. (2012). Cereal grains for nutrition and health benefits: overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Technol 25(2):87–100.
- Bove, J.; Jullien, M. and Grappin, P. (2001). Functional genomics in the study of seed germination. Genome Biology, 3(1), reviews1002.1.
- Buschmann, M. (2018). Diversity of sorghum. In Second European Sorghum Congress. Origin in Northeastern Africa as a wild plant
- Cevallos-Casals, B.A. and Cisneros-Zevallos, L. (2010). Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem., 119(4):1485–1490.
- Daddario, J.F.F.; Bentivegna, D.J.; Tucat, G. and Fernandez, O.A. (2017). Environmental factors affecting seed germination of common teasel (Dipsacus fullonum). Planta Daninha, 35: e017163536.
- Delcour, J.A.; Rouau, X.; Courtin, C.M.; Poutanen, K. and Ranieri, R. (2012). Technologies for enhanced exploitation of the health-promoting potential of cereals. Trends Food Sci., Technol. 25(2):78–86.
- Demeke, T.; Chang, H.G. and Morris, C.F. (2001). Effect of germination, seed abrasion and seed size on polyphenol oxidase assay activity in wheat. Plant Breed 120(5):369–373.
- Desai, R.P.; Dave., D.; Suthar, S.A.; Shah, S.; Ruparelia, N. and Kikani, B.A. (2021). Immobilization of α-amylase on GO-magnetite nanoparticles for the production of high maltose containing syrup. International Journal of Biological Macromolecules, 169: 228-238.
- Dicko, M.H.; Gruppen, H.; Traoré, A.S.; van Berkel, W.J. and Voragen, A.G. (2005). Evaluation of the effect of germination on phenolic compounds and antioxidant activities in sorghum varieties. J. Agric. Food Chem. 53(7):2581–2588.
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J. and Vasiljevic, T. (2012). Germinated grains–sources of bioactive compounds. Food Chem. 135(3):950–959.
- Egli, I.; Davidsson, L.; Juillerat, M.A.; Barclay, D. and Hurrell, R.F. (2002). The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feedin. J. Food Sci. 67(9):3484–3488.
- El-Naim, A.M.; Mohammed, K.E.; Ibrahim, E.A. and Suleiman, N.N. (2012). Impact of salinity on seed germination and early seedling growth of three sorghum (Sorghum biolor L. Moench) cultivars. Science and Technology, 2(2): 16-20.
- Fardet, A.; Rock, E. and Rémésy, C. (2008). Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? J. Cereal Sci., 48(2):258–276.
- Fardet, A. (2010). New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nut. Res. Rev., 23(1):65–134.
- Food, U.S. (2012). Drug Administration (US FDA) National nutrient database for standard reference Release 24. Retrieved 10 September 2012, from http://www.ars.usda.gov/main/site_main. htm?modecode=12-35-45-00.
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q. and Li, H.B. (2011). Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem., 129(2):345–350.
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q. and Corke, H., H. (2017). Bioactive compounds and bioactivities of germinated edible seeds and sprouts: an updated review. Trends Food Sci. Technol., 59:1–14.
- Garriguet, D. (2007). Canadians’ eating habits. Health Rep 18(2):17.
- Girma, F.; Mekbib, F.; Tadesse, T.; Menamo, T. and Bantte, K. (2020). Phenotyping sorghum Sorghum bicolor (L.) Moench for drought tolerance with special emphasis to root angle. African Journal of Agricultural Research, 16(8), 1213-1222.
- Go Grains Health and Nutrition (GGHN). (2010). The grains and legumes health report: a review of the science. Spit Point, NSW 2088: Go Grains Health and Nutrition Grindberg RV, Ishoey T, Brinza D, Esquenazi E, Coates RC, Liu WT.
- Grindberg, R.V.; Ishoey, T.; Brinza, D.; Esquenazi, E.; Coates, R.C.; Liu, W.T. and Gerwick W.H. (2011). Single cell genome amplification accelerates identification of the apratoxin biosynthetic pathway from a complex microbial assemblage. PLoS One 6(4):e18565.
- Guajardo-Flores, D.; Serna-Saldívar, S.O. and Gutiérrez-Uribe, J.A. (2013). Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.). Food Chem., 141(2):1497–1503.
- Guo, X.; Li, T.; Tang, K. and Liu, R.H. (2012). Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem., 60(44):11050–11055.
- Gupta, N.; Beliya, E.; Paul, J.S.; Tiwari, S.; Kunjam, S. and Jadhav, S.K. (2021). Molecular strategies to enhance stability and catalysis of extremophile-derived α-amylase using Computational Biology. Extremophiles, 25 (3): 221-233.
- Gupta, R.K.; Gangoliya, S.S. and Singh, N.K. (2015). Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci., 52(2):676–684.
- Hassan, S.; Hussain, M. B.; Waheed, M.; Ahmad, K.; Kassymov, S.; Shariati, M. A. and Egbuna, C. (2020). Effect of Germination Processing on Bioactive Compounds of Cereals and Legumes. Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations, 283-306.
- Hassouni, A. A. (2019). The effect of irrigation periods on the growth and yield of Sorghum cultivars, and accompanying bushes. Master’s thesis, Department of Field Crops, College of Agriculture, University of Basra, pp. 1-128.
- Hefni, M. and Witthöft, C.M. (2011). Increasing the folate content in Egyptian baladi bread using germinated wheat flour. LWT., 44(3):706–712.
- Hemalatha, S.; Platel, K. and Srinivasan, K. (2007). Influence of germination and fermentation on bioaccessibility of zinc and iron from food grains. Eur. J. Clin. Nut., 61(3):342.
- Hübner, F. and Arendt, E.K. (2013). Germination of cereal grains as a way to improve the nutritional value: a review. Critical Rev. Food Sci. Nut., 53(8):853–861.
- Hung, P.V.; Maeda, T.; Yamamoto, S. and Morita, N. (2012). Effects of germination on nutritional composition of waxy wheat. J. Sci. Food Agric., 92(3):667–672.
- Hussain, M.S.; Fareed, S.; Saba, A. M; Rahman, A.; Ahmad, I.Z. and Saeed M. (2012). Current approaches toward production of secondary plant metabolites. J. Pharm Bioallied Sci., 4(1):10.
- Imam, M.U.; Musa, S.N.A.; Azmi, N.H. and Ismail, M. (2012). Effects of white rice, brown rice and germinated brown rice on antioxidant status of type 2 diabetic rats. Int. J. Mol. Sci., 13(10):12952–12969.
- Iordan, M.; Stoica, A. and Popescu, E.C. (2013). Changes in quality indices of wheat bread enriched with biologically active preparations. Annals Food Sci. Technol., 14:165–170.
- Johansson, E.; Prieto-Linde, M.L. and Larsson, H. (2021). Locally adapted and organically grown landrace and ancient spring cereal. A unique source of minerals in the human diet. Foods, 10(2):393.
- Jonnalagadda, S.S.; Harnack, L.; Hai Liu, R.; McKeown, N.; Seal, C.; Liu, S.; Fahey, G.C. (2011). Putting the whole grain puzzle together: health benefits associated with whole grains—summary of American Society for Nutrition 2010 satellite symposium. J. Nutr., 141(5):1011S–1022S.
- Kandil, A.A.; Sharief, A.E.; Seadh, S.E.; Alhamery, J.I.K. (2015). Germination parameters enhancement of maize grain with soaking in some natural and artificial substances. J. Crop Sci., 6(1):142–149.
- Katami, K. (2004). Chemical analysis of seeds of Sorghum cultivars grown in different locations in Basra, 1(7), Basra Journal of Agricultural Sciences.
- Kaukovirta-Norja, A.; Wilhelmson, A. and Poutanen, K. (2004). Germination: a means to improve the functionality of oat. Agric. Food Sci., 13(1-2):100–112.
- Kim, Y.B.; Thwe, A.A.; Kim, Y.; Yeo, S.K.; Lee, C. and Park, S.U. (2013). Characterization of cDNA encoding resveratrol synthase and accumulation of resveratrol in tartary buckwheat. Natural Product Communications,
8(11):1571–1574. - Koehler, P.; Hartmann, G.; Wieser, H. and Rychlik, M. (2007). Changes of folates, dietary fiber, and proteins in wheat as affected by germination. J. Agric. Food Chem. 55(12):4678–4683.
- Krzyzanowska, J.; Czubacka, A. and Oleszek, W. (2010). Dietary phytochemicals and human health. In: Bio-farms for nutraceuticals. Springer, Boston, pp. 74–98.
- Layek, J.; Das, A.; Mitran, T.; Nath, C.; Meena, R. S.; Yadav, G. S. and Lal, R. (2018). Cereal+ legume intercropping: An option for improving productivity and sustaining soil health. In Legumes for Soil Health and Sustainable Management (pp. 347-386). Springer, Singapore.
- Limón, R.I.; Peñas, E.; Martínez-Villaluenga, C. and Frias J. (2014). Role of elicitation on the health promoting properties of kidney bean sprouts. LWT-Food Science and Technology, 56(2):328–334.
- Liukkonen, K.H.; Katina, K.; Wilhelmsson, A.; Myllymaki, O.; Lampi, A.M.; Kariluoto, S. and Peltoketo A, A. (2003). Process-induced changes on bioactive compounds in whole grain rye. Proc Nutr. Soc
62(1):117–122. - Mak, Y.; Willows, R.D.; Roberts, T.H.; Wrigley, C.W; Sharp, P.J. and Copeland, L. (2009). Germination of wheat: a functional proteomics analysis of the embryo. Cereal Chem., 86(3):281–289.
- Mäkinen, O.E. and Arendt, E.K. (2015). Nonbrewing applications of malted cereals, pseudocereals, and legumes: A review. J. of the American Society of Brewing Chemists, 73(3): 223-227.
- Mardian, I. (2020). Performance and utilization of local sorghum (Sorghum bicolor L.) in West Nusa Tenggara. In IOP Conference Series: Earth and Environmental Science, 484 (1): p. 012092). IOP.Publishing
- Marton, M.; Mandoki, Z.S.; Csapo-Kiss, Z.S. and Csapo, J. (2010). The role of sprouts in human nutrition. A review. Acta Univ Sapientiae 3:81–117.
- Masters, R.C.; Liese, A.D.; Haffner, S.M.; Wagenknecht, L.E. and Hanley, A.J. (2010). Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J. Nutr., 140(3):587–594.
- Mateo Anson, N.; Aura, A.M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; Van den Berg, R. and Haenen, G.R. (2010). Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts anti-inflammatory effects ex vivo–3. J. Nutr. 141(1):137–143.
- Matpan Beckler, F.; Güven, K. and Gül Güven, R. (2021). Purification and characterization of novel α-amylase from Anoxybacillus ayderensis FMB1. Biocatalysis and Biotransformation, 39(4), 322-332.
- Matsa, M.; Mavugara, R. and Dzawanda, B. (2021). Urban domestic water supply crisis in the city of Gweru, Zimbabwe. Geo J., 86(3): 1173-1192.
- Mihafu, F.; Laswai, H.S.; Gichuhi, P.; Mwanyika, S. and Bovell-Benjamin, A.C. (2017). Influence of soaking and germination on the iron, phytate and phenolic contents of maize used for complementary feeding in rural Tanzania. Int. J. Nut. Food Sci., 6(2):111–117.
- Miransari, M. and Smith, D.L. (2014). Plant hormones and seed germination. Environmental and experimental botany, 99:110–121.
- Missarah, M.J.; Ibrahim, I.; Hamid, A.A. and Aqma, W.S. (2020). Optimization and production of alpha amylase from thermophilic Bacillus spp. And its application in food waste biodegradation. Heliyon, 6(6): e04183.
- Mokhtarpour, A.; Naserian, A.A.; Valizadeh, R.; Mesgaran, M.D. and Pourmollae, F. (2014). Extraction of phenolic compounds and tannins from pistachio by-products. Annual Research and Review in Biology, 1330-1338.
- Moongngarm, A. and Saetung, N. (2010). Comparison of chemical ompositions and bioactive compounds of germinated rough rice and brown rice. Food Chem., 122(3):782–788.
- Nam, T.G.; Lee, S.M.; Park, J.H.; Kim, D.O.; Baek, N.I. and Eom, S.H. (2015). Flavonoid analysis of buckwheat sprouts. Food Chem., 170:97–101.
- Nelson, K.; Stojanovska, L.; Vasiljevic, T. and Mathai, M. (2013). Germinated grains: a superior whole grain functional food? Can J. Physiol, Pharmacol, 91(6):429–441.
- Newby, P.K.; Maras, J.; Bakun, P.; Muller, D.; Ferrucci, L. and Tucker, K.I. (2007). Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J. Clin Nut. 86(6):1745–1753.
- Noda, T.; Takigawa, S.; Matsuura-Endo, C.; Saito, K.; Takata, K.; Tabiki, T. and Yamauchi, H. (2004). The physicochemical properties of partially digested starch from sprouted wheat grain. Carbohydrate
Polymers 56(3):271–277. - Nour, A.A.M.; Ibrahim, M.A.E.M.; Abdelrahman, E.E.; Osman, E.F. and Khadir, K.E. (2015). Effect of processing methods on nutritional value of sorghum (Sorghum bicolor L. Moench) cultivar. Am J. Food Sci. Health 1(4):104–108.
- Nyoni, N.; Dube, M.; Bhebhe, S.; Sibanda, B.; Maphosa, M. and Bombom, A. (2020). Understanding biodiversity in sorghums to support the development of high value bio-based products in sub-Saharan Africa. Journal of Cereals and Oilseeds, 11(2): 37-43.
- Ojo, O.I.; Ogunlade, I. and Adeyeye, E. (2020). Comparative study of effect of sprouting on water and fat soluble vitamins in white sorghum bicolor and pennisetum glau. IOSR, Journal of Environmental Science, Toxicology and Food Technology, 14(1): 15-21.
- Okarter, N. and Liu, R.H. (2010). Health benefits of whole grain phytochemicals. Critical Rev. Food Sci. Nut., 50(3):193–208.
- Ozturk, I.; Sagdic, O.; Hayta, M. and Yetim, H. (2012). Alteration in α-tocopherol, some minerals, and fatty acid contents of wheat through sprouting. Chem. Nat. Compounds 47(6):876–879.
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J. and Fortuna, T. (2014). Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 143:300–306.
- Panfil, P.; Dorica, B.; Sorin, C.; Emilian, A.E. and Iosif, G. (2014). Biochemical characterization of flour obtained from germinated cereals (wheat, barley and oat). Rom Biotech Lett, 19(5):9773.
- Parsaeimehr, A.; Sargsyan, E.; Vardanyan, A. (2011). Expression of secondary metabolites in plants and their useful perspective in animal health.
- Pawar, V.D.; Machewad, G.M. and G. M. (2006). Changes in availability of iron in barley during malting. J. Food Sci. Technol., 43(1):28–30.
- Poutanen, K. (1997). Enzymes: an important tool in the improvement of the quality of cereal foods. Trends Food Sci. Technol., 8(9):300–306.
- Rakcejeva, T.; Zagorska, J. and Zvezdina, E. (2014). Gassy ozone effect on quality parameters of flaxes made from biologically activated whole wheat grains. International Journal of Nutrition and Food Engineering, 8(4):396–399.
- Rana, J. and Paul, J. (2020). Health motive and the purchase of organic food: A meta – analytic review. International Journal of Consumer Studies, 44(2): 162-171.
- Rani, K.U.; Rao, U.P.; Leelavathi, K. and Rao, P.H. (2011). Distribution of enzymes in wheat flour mill streams. J. Cereal Sci., 34(3):233–242.
- Saini, A.; Panwar, D.; Panesar, P.S. and Bera, M.B. (2019). Bioactive compounds from cereal and pulse processing by-products and their potential health benefits. Austin J. Nutr. Metab., 6(2): 1068.
- Saini, R.; Saini, H.S. and Dahiya, A. (2017). Amylases: Characteristics and industrial applications. Journal of Pharmacognosy and Phytochemistry, 6(4): 1865-1871.
- Sangronis, E. and Machado, C.J. (2007). Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. LWT 40(1):116–120.
- Šēnhofa, S.; Zeps, M.; Gailis, A.; Kāpostiņš, R. and Jansons, Ā. (2016). Development of stem cracks in young hybrid aspen plants. Forestry Studies, 65(1):16.
- Sghaier-Hammami, B.B.M.; Hammami, S.; Baazaoui, N.; Gómez-Díaz, C. and Jorrín-Novo, J.V. (2020). Dissecting the seed maturation and germination processes in the Non-Orthodox Quercus ilex species based on protein signatures as revealed by 2-DE coupled to MALDI-TOF/TOF proteomics strategy. International Journal of Molecular Sci., 21(14): 4870.
- Shohag, M.J.I.; Wei, Y. and Yang, X. (2012). Changes of folate and other potential health-promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem., 60(36):9137–9143.
- Skoglund, M.; Peterson, D.M.; Andersson, R.; Nilsson, J. and Dimberg, L.H. (2008). Avenanthramide content and related enzyme activities in oats as affected by steeping and germination. J. Cereal Sci., 48(2):294–303.
- Sohail, S.; Ansar, M.; Skalicky, M.; Wasaya, A.; Soufan, W.; Ahmad Yasir, T. and EL Sabagh, A. (2021). Influence of tillage systems and cereals-legume mixture on fodder yield, quality and net returns under rainfed conditions. Sustainability, 13(4): 2172.
- Stagnari, F.; Maggio, A.; Galieni, A. and Pisante, M. (2017). Multiple benefits of legumes for agriculture sustainability: an overview. Chemical and Biological Technologies in Agriculture, 4(1): 1-13.
- Sudha, R. R. and Usha, A. (2014). Effect of germination and fermentation on polyphenols in finger millet (Eleusine coracana). Int. J. Food Nut. Sci., 3:65–68.
- Tang, D.; Dong, Y.; Guo, N.; Li, L. and Ren, H. (2014). Metabolomic analysis of the polyphenols in germinating mung beans (Vigna radiata) seeds and sprouts. J. Sci. Food Agric., 94(8):1639–1647.
- Teresa, T.; Bejiga, T.; Semahegn, Z.; Seyoum, A.; Kinfe, H.; Nega, A. and Ayalew, T. (2021). Evaluation of advanced sorghum (Sorghum bicolor L. Moench) hybrid genotypes for grain yield in moisture stressed areas of Ethiopia.
- Theodoulou, F.L. and Eastmond, P.J. (2012). Seed storage oil catabolism: a story of give and take. Curr Opin Plant Biol., 15(3):322–328.
- Tobias, J.R.; Castro, I.J.L.; Peñarubia, O.R.; Adona, C.E. and Castante, R.B. (2018). Physicochemical and functional properties determination of flour, unmodified starch and acid-modified starch of Philippine-grown sorghum (Sorghum bicolor L. Moench). International Food Research Journal, 25(6): 2640-2649.
- Trabelsi, S.; Ben Mabrouk, S.; Kriaa, M.; Ameri, R.; Sahnoun, M.; Mezghani, M. and Bejar, S. (2019). The optimized production, purification, characterization, and application in the bread making industry of three acid-stable alpha-amylase isoforms from a new isolated Bacillus subtilis strain US586. Journal Food Biochem., 43(5): e12826.
- Van Hung, P.; Hatcher, D.W. and Barker, W. (2011). Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem., 126(4):1896–1901.
- Wang, T.; He, F. and Chen, G. (2014). Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: a concise review. J. Funct Foods, 7:101–111.
- Williams, J.A. (2019). Amylase. Pancreapedia: The Exocrine Pancreas Knowledge Base:1-8.
- Xiong, Y.; Zhang, P.; Warner, R.D. and Fang, Z. (2019). Sorghum grain: from its genotype, nutrition, and phenolic profile to its health benefits and food applications. Comprehensive Reviews in Food Science and Food Safety, 18(6): 2025-2046.
- Xue, Z.; Wang, C.; Zhai, L.; Yu, W.; Chang, H.; Kou, X. and Zhou, F. (2016). Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.), soybean (Glycine max L.) and black bean
(Phaseolus vulgaris L.) during the germination process. Czech J. Food Sci. 34(1):68–78 - Yang, T.K.; Basu, B. and Ooraikul, F. (2001). Studies on germination conditions and antioxidant contents of wheat grain. Int. J. Food Sci. Nutr., 52(4):319–330.
- Zamora-Ros, R.; Knaze, V.; Rothwell, JA; Hemon, B.; Moskal, A.; Overvad, K. and Scalbert, A. (2016). Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. European Journal of Nutrition, 55(4): 1359-1375.
- Zhao, Y.; Jiang, Q. and Wang, Z. (2019). The System Evaluation of Grain Production Efficiency and Analysis of Driving Factors in Heilongjiang Province. Water, 11(5): 1073.
- Zieliński, H. and Kozłowska, H. (2000). Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem., 48(6):2008–2016.
- Zilic, S.; Basic, Z.,; Sukalovic, V.H.T.; Maksimovic, V.; Jankovic, M. and Filipovic, M. (2014). Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and
antioxidant capacity of the flour? Int. J. Food Sci. Technol., 49:1040–1047.

