Studying the effect of temperature on the structural and morphological characterization of silver nano-partical using moringa extract
Ban M.A Alani1, Rawia Abdelgain Elobaid Mohammed2 ,Harakat Mohsin Roomy3
1College of Medicine, university of Fallujah, Iraq
2College of science, university of Sudan of science and technology, Sudan
3Department of physics, Ministry of Education, Baghdad, Iraq
HNSJ, 2024, 5(8); https://doi.org/10.53796/hnsj58/2
Published at 01/08/2024 Accepted at 05/07/2024
Abstract
Abstract. The moringa extract leaves extract has been used for green synthesis of silver nanoparticles. The impact of temperature on the production of silver nanoparticles and the reduction of silver ions was studied. Applying heat improves green synthesis, where at a relatively low temperature of 40 °C, it was clear that silver ion reduction occurred rapidly by the appearance of a strong surface plasmon resonance (SPR) peak at around 430 nm according to analysis of the ultraviolet and visible spectra in compared to the other temperatures that were under study (60 and 80ºC). whereas higher temperatures (60,80°C) are necessary for the efficient production of silver nanoparticles, where smaller nanoparticles are formed as a result of the reactants being rapidly consumed at higher temperatures. Also it was found that the UV spectrum showed that a rise in temperature can cause a variation in the shape and position of peak formation around 430 nm. Analysis using X-ray diffraction confirmed that the produced solutions included silver nanoparticles with a crystalline structure in the face-centered cubic (FCC) phase. As the temperature increased from 40 to 80 ºC, the crystals saw a decreases in average particle size. The growth mechanism and aggregation process are affected by temperature changes, as demonstrated by the SEM results, with silver nanoparticles assuming mostly spherical shapes with different dimensions depending on the temperature at which the reaction occurs.
Key Words: Green synthesis; Silver Nanoparticles; Plasmon resonance; temperature
1. Introduction
Nowadays, nanoscience is a prominent field of study that provides opportunities for basic and practical research in all areas of cognitive science. Numerous properties set nanomaterials apart from their bulk counterparts, such as size, visual effects, chemical reactivity, physical strength, electrical conductance, and magnetism [1]. Due to the wide range of possible applications in biological, optical, and electrical fields, nanoparticle research is currently a highly pursued scientific field. Between bulk materials and molecular (atomic) structures, nanoparticles provide a thin bridge [2]. Nanoscale materials consist of a single grain with all atoms oriented in a crystal lattice, while bulk materials contain random grains that are independently oriented in space and touch each other across grain boundaries. For this reason, the physical properties of bulk materials remain constant [3]. Although chemical and physical methods are highly effective in producing well-defined nanoparticles, they have certain disadvantages, including high production costs, release of toxic byproducts, long synthesis times, and difficult purification [4]. Due to the growing awareness of the need to dispose of toxic and hazardous waste caused by climate change and global warming, the green synthesis approach has led to significant advances in scientific research [5]. As the name suggests, nanoparticle biosynthesis helps synthesize very complex reactions within minutes, and this has drawn attention to the requirement of environmentally friendly technologies in materials science [6]. The unique qualities of silver nanoparticles, including high conductivity, chemical stability, catalytic activity, surface-enhanced Raman scattering, and antibacterial activity, have attracted great interest in the field of nanotechnology research [7]. Silver is a common catalyst for the oxidation of methanol to ethylene oxide and formaldehyde. Because it partly requires an electrically conductive surface, its colloidal nature makes it useful as a substrate for surface-enhanced spectroscopy. Silver is a popular antibacterial agent in modern times. Recently, there has been a focus on the fabrication of silver nanoparticles to combat antibiotic resistance arising from drug use [8]. Green synthesis of AgNPs using different biological agents such as plants, bacteria, fungi, algae and yeast is an economical, easy and environmentally friendly method without generating any toxic byproducts. In recent years, both microbes and plants have been extensively investigated for the green synthesis of AgNPs[9]. A reducing biological agent and a silver metal ion solution are essential for the green synthesis of AgNPs. There is typically no need to add external capping and stabilizing agents because reducing agents or other components already present in the cells function as these substances’ stabilizing and capping agents [10]. The use of plants for nanoparticle synthesis could have an advantage over other biological processes by eliminating the complex process of maintaining cell cultures [11]. Moringa leaves have medicinal properties as the plant extracts of these leaves have shown strong antioxidant and antibacterial properties against both Gram-positive and Gram-negative bacteria [12]. The aim of this study the investigation of oleifera leaf extract use in the synthesis of AgNPs and to examine the effect of temperature on the structural, optical and morphological characterization of silver nano-partical using moringa extract obtained by chemical reduction of AgNO3
2- Materials and Methods
2-1 Preparation plant extract of M. oleifera leaf
Following collection and washing in double distilled water to remove any dust, moringa leaves were dried in a dark room for two weeks before being ground into a powder. After drying, 10 g of each sample was ground into powder using a mortar and pestle and blender, and then added to 100 mL of distilled water using a magnetic stirrer for 1 h at room temperature. The mixtures were then stirred for an entire day on an orbital shaker. The filtrate is taken and then sterilized using 0.4 micrometer bacterial filters. and the clear solution is placed. In the dryer, at a temperature of 35 °C until the extract dries, then a weight of (1 gram) is taken from the dry extract, and the volume is added to 100 ml of distilled water as shown in Figure1.Thus, a stock solution with a concentration of 10,000 ppm is obtained, which is mixed with a magnetic mixer for 15 minutes until dissolution. Complete the extract and from this solution the rest is prepared concentration.
 Figure 1 preparation processes of extract of M. oleifera leaf
Figure 1 preparation processes of extract of M. oleifera leaf
2.2-Synthesis of silver nanoparticles
For preparation (1) ml of silver nanoparticles (Ag NPs), a 4 mM silver nitrate (AgNO3) solution with a capacity of 100 mL was prepared. Then, at room temperature (28.0 ± 2.0 °C), 5 ml of AgNO3 solution add to 1 ml of M. oleifera, then reduce the mixed solution in 100 ml of distilled water. After that, the mixture was left overnight at 30°C on a magnetic stirrer rotating at 150 rpm. And then the plant extract, reduction of silver nitrate to silver nanoparticles, and color change from yellow to red then brown in aqueous solution. Silver nanoparticles were prepared by varying the temperature. Where 10 ml of silver nitrate was added to the concentration obtained in the first stage to 1 ml of Moringa plant at a temperature of 20oC. This mixing process was repeated for several more degrees (40, 60, and 80) oC. Then we take the best nano size at the best temperature by conducting tests (FESEM, XRD, FTIR, and UV-Vis. ) for each temperature.

2-3 Characterization of silver nanoparticles
A Japanese-made SHIMADZU UV-2600 UV-Vis spectrometer was used to record the absorbance spectrum (190-1100 nm) of the synthesized silver nanoparticles. The phase purity of synthesized silver nanoparticles was determined using XRD analysis (SHIMADZU Japan) 40 kV. Current: 30 mA. Speed: 8 deg /min. Range (10 – 80oC). FTIR spectroscopy (FT-IR Shimadzu-8400 max resolution 4cm-1, S/N ratio: 30000:1, the spectra range is 4000-400 cm-1) provides information on the functional groups present in nanoparticles, enabling us to determine the transformation of silver nanoparticles from the inorganic compound AgNO3 to elemental silver. The spectra were scanned at a resolution of 4 cm−1 throughout the range of 4000–400 cm−1. To carefully examine the very fine topography a field emission scanning electron microscope (FESEM) instrument was used.
3. Results and discussion
UV-vis spectroscopy was used in this study to observe the bio reduction of silver ions to AgNPs. Measurements of UV-Vis absorption in the 300–800 nm region can offer a thorough understanding of the optical characteristics of the generated nano sized silver particles. Figure 1 displays the ultraviolet (UV) absorption peaks of silver nanoparticles (Ag NPs) synthesized by M. oleifera at several temperatures (40, 60, and 80 °C). This figure shows that, the changes in the intensity of the SP resonance band are strongly affected by the synthesis temperature. The solution combination at temperatures of 60 and 80 °C exhibited a little peak (311.08, 298.2 nm) respectively, but the UV-vis spectra at 40 °C had a strong surface Plasmon resonance (SPR) peak at around 430 nm. UV spectra showed that at lower temperatures, the wavelength was higher, Conversely, at higher temperatures, the wavelength changed to a lower value, which led to the synthesis of smaller silver nanoparticles. It indicates that reactants are quickly consumed at higher temperatures, leading to the creation of smaller nanoparticles [13].
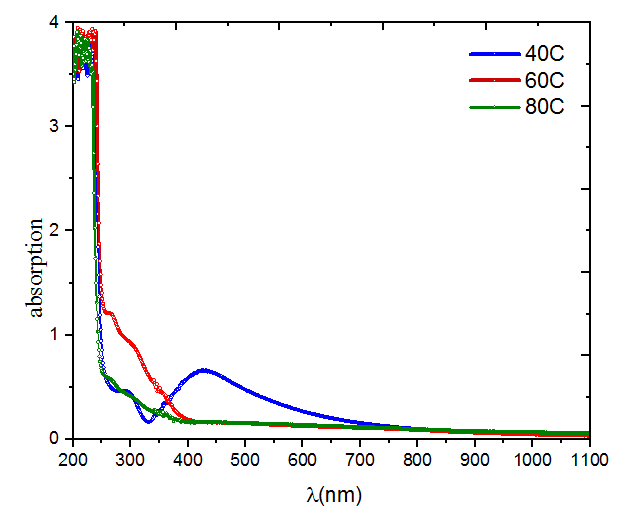
Figure 1 UV-visible absorption peak of M. Oliefera synthesized silver nanoparticles at different temperature
Figure 2 displays XRD patterns of AgNp thin layer prepared using the process of green synthesis and deposited by drop casting on glass substrates. The X-ray diffraction pattern of the Ag nanoparticles generated at various temperatures is shown in the Figure, along with the corresponding data in Table 5.2. The sample (40oC) exhibited many Bragg reflection peaks with 2θ values and planes; 29.386o (210), 32.3591o (122), 34.754o(111), 41.873o(200), and 48.270o (231) as determined by indexing based on the face-centered cubic structure. The sample was measured at a temperature of 60 oC. The values of 2θ were found to be 38.278o (111), 44.447o (200) 64.614o(220) and 77.578o(311) of the face centered cubic (fcc) crystal structure of Ag NPs. While the measured 2θ angles for the sample at a temperature of 80°C were 29.56°(210), 32.76°(122), and 35.42°(111) of the face centered cubic (fcc) crystal structure of Ag NPs. The indexing was done using the Standard on Powder Diffraction data (JCPDS, file No. 04-0783). There are additional peaks found before the angle 28º, which are caused by the phytochemical components present in the extract. These chemicals are responsible for decreasing the silver ions, and this result is in agreement with previous studied [14]. The XRD data unambiguously demonstrated the crystalline character of the Ag nanoparticles produced using the extract. The average size of the Ag nanoparticles formed is around 54.75 nm, 45.809 nm, and 35.99 nm at temperatures of 40°C, 60°C, and 80°C, respectively, the crystalline size reduced as the temperature increased as shown in table 1, and this result is consistent with the analyzes of the UV-Vis spectra. This is attributed to the completion of the silver ion reduction process, which reduces the agglomeration of the nanoparticles and increases their diffusion, thus increasing the synthetic stress that Nano-crystals suffer, which causes the lattice to shrink and the crystal size decreases accordingly, and this is entirely consistent with the results obtained from ref. [15].
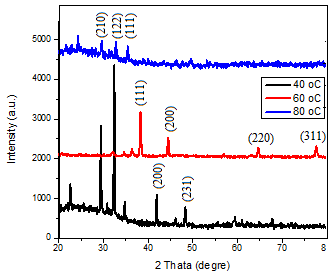
Figure 2
XRD patterns of Ag NPs prepared at different temperature.
Table 1 The stricture parameter of Ag NPS at different temperature.
The figure 3 shows the variations in particle sizes, forms, and morphologies of Ag NPs which formed on the surface of M. oliefera at various temperatures (40, 60, and 80oC). The sizes of the silver nanoparticles ranged from 12 to 103 nm, were spherical in shape and highly dispersed for different temperatures. We observed a particle size of 12.05 nm at 40 °C, 31.13 nm at 60 °C, and 103 nm at 80 °C.
In other words, when temperature increases, the size of particles increases. As the reaction temperature rises, the AgNO3 molecules might undergo degradation, resulting in a reduction in size of the silver nanoparticles. Consequently, the nanoparticles exhibit enhanced efficacy. As the temperature rises, the size of the particles increases, resulting in a smaller variety of sizes [16]
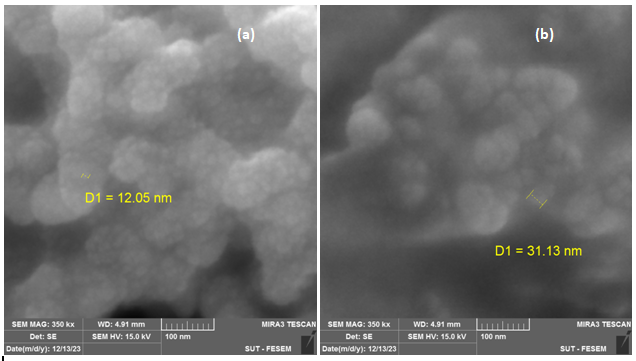

Figure 3 SEM images of AgNPs with M. oleifera at different temperature (a)40 oC, (b)60 oC, (c) 80 oC.
we conducted FTIR analysis on AgNO3 solutions with concentrations 4 mol at (40ºC) as optimal temperature. Functional groups have been seen in the 500–4000 cm-1 range. As shown in the figure 4, FTIR analysis showed significant peaks (3402.43, 1377.17, 823.6) cm-1, The peaks seen at 3402.43 and 3415.93 cm-1 are associated with the stretching vibrations of the hydroxyl (OH) functional group, which is found in alcohols and phenolic compounds. The presence of carbonyl groups (C=O) in protein amides I and II may explain the observed peaks at 1377.17, 1693.49, 1414.00, and 1382.96 cm-1. In addition, the stretching vibration band of the C=O group and the curving vibration band of the OH group are used in the synthesis of AgNPs as a reducing, capping, and stabilizing agent [17]. The presence of a peak at 823.6 cm-1 suggests that the elongation of the C-O bond might perhaps be attributed to the presence of an ether compound [18].
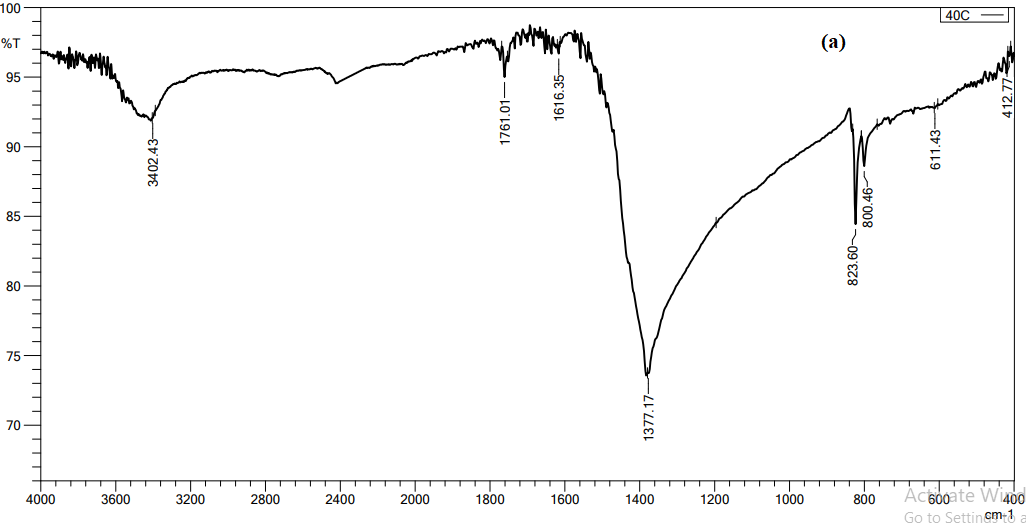
Figure 4 FTIR spectroscopy analysis of AgNPS with M. oleifera, at optimal temperature 40 ºC
Figure 5 confirms that the Ag NPs produced are mostly composed of silver nanoparticles. Whereas, according to the energy dispersive X-ray (EDX) result, most of the major components showed intense peaks of Ag (76.23%) at 3.0 Kev and O (23. 77%).These components likely play a role in stabilizing plant extracts and encapsulating biomolecules; In addition, they may be related to components found in plant proteins
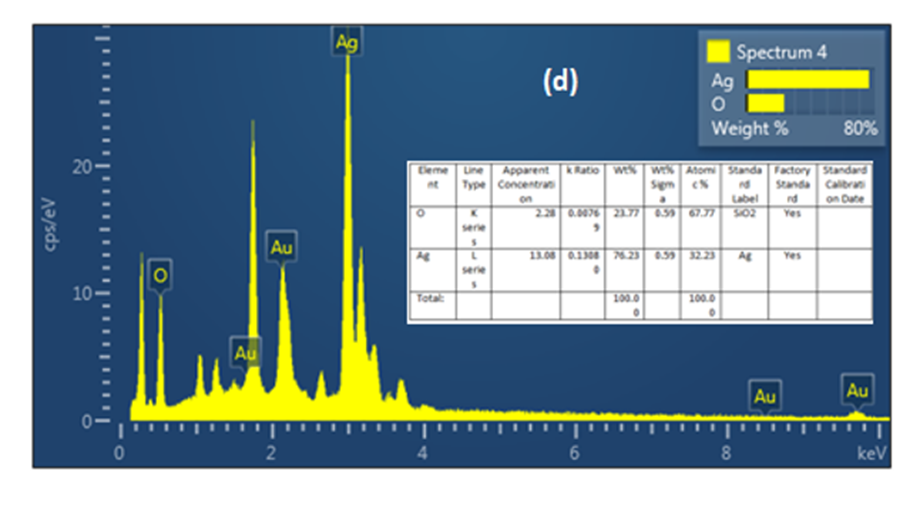
(Figure 5) Energy dispersive x-ray (EDX) analysis of AgNPs with M. oleifera extracts at 40ºC
4. Conclusion
The fabrication of metal nanoparticles using aqueous plant extracts is taking importance over traditional methods of synthesis, especially due to their ease of preparation, low cost and low toxicity. The aim of this study, the changing of temperature impact on green synthesis of the silver nanoparticle synthesized from the moringa extract. UV spectrum showed that, the changes in the intensity of the SP resonance band are strongly affected by the synthesis temperature. The solution combination at temperatures of 60 and 80 °C exhibited a little peak (311.08, 298.2 nm) respectively, but the UV-vis spectra at 40 °C had a strong surface Plasmon resonance (SPR) peak at around 430 nm.The XRD data unambiguously demonstrated the crystalline character of the Ag nanoparticles produced using the extract. The average size of the Ag nanoparticles formed is around 54.75 nm, 45.809 nm, and 35.99 nm at temperatures of 40°C, 60°C, and 80°C, respectively. the higher degree in temperature, the smaller the size of the AgNPs produced. Therefore, it is possible to control the crystalline size of nanoparticles by controlling preparation conditions such as temperature and plant type. Also, according to the (EDX) result, most of the major components showed intense peaks of Ag (76.23%) at 3.0 keV and O (23. 77%). These substances probably stabilize plant extracts and encapsulate biomolecules; they might also be connected to substances present in plant proteins.
Reference
[1] Madeeha Ansari, Shakil Ahmed, Asim Abbasi, Muhammad Tajammal Khan, Mishal Subhan, Najat A. Bukhari, Ashraf Atef Hatamleh & Nader R. Abdelsalam, Plant mediated fabrication of silver nanoparticles, process optimization, and impact on tomato plant, Scientific Reports volume 13, Article number: 18048 (2023).
[2] Matussin, S., Harunsani ,M.H., Tan, A.L., Khan ,M.M.,(2020)’Plant-extract- mediated SnO2 nanoparticles: synthesis and applications’, Sustain Chem Eng,8(8),PP.3040-3054.
[3] Sharma KV, Yngard AR, Lin Y, (2009). Silver nanoparticle: Green synthesis and their antimicrobial activities, Advances in Colloid and Interface Science 145 83–96.
[4] Nagajyothi PC and Lee KD, (2011). Synthesis of Plant-Mediated Silver Nanoparticles Using Dioscorea batatas Rhizome Extract and Evaluation of Their Antimicrobial Activities. Journal of Nanomaterials, 573429.
[5] Ahmad N, Sharma S, Singh V. N., Shamsi S. F., Fatma A, Mehta B. R, (2011). Biosynthesis of Silver Nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnology Research International Volume.
[6] Harekrishna, B., Dipak, K. B., Gobinda, P. S., Priyanka, S., Santanu, P., & Ajay, M. (2009). Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A Physicochem Eng Asp, 348(1-3), 212-216.
[7] Setua P, Chakraborty A, Seth D, Bhatta MU, Satyam PV, Sarkar N, (2007). Synthesis, optical properties, and surface enhanced Raman scattering of silver nanoparticles in nonaqueous methanol reverse micelles, J. Phys. Chem. C., 111, 3901-3907.
[8] Panaek A, Kvitek L, Prucek R, Kolar M, Veerova R, Pizurova N, Sharma VK, Nevena T and Zboril R, (2006).Silver colloid nanoparticles: Synthesis, characterization and their antibacterial activity. J. Phys. Chem. B 110, 16248–16253.
[9]F. Kanjirathamthadathil ,A. Mathew, A. Parveen, V. Valiyathra, G. Vazhathara , Novel green synthesis of silver nanoparticles using clammy cherry (Cordia obliqua Willd) fruit extract and investigation on its catalytic and antimicrobial properties. SN Applied Sciences (2019) 1:1368.
[10] S. Iravani, H. Korbekandi, S.V. Mirmohammadi, and B. Zolfaghari, Synthesis of silver nanoparticles: chemical, physical and biological methods, Res Pharm Sci. 2014 Nov-Dec; 9(6): 385–406.
[11] Francis S, Joseph S, Koshy EP, Mathew B (2017) Synthesis and characterization of multifunctional gold and silver nanoparticles using leaf extract of: Naregamia alata and their applications in the catalysis and control of mastitis. New J Chem 41:14288–14298.
[12] Mmabatho Kgongoane Segwatibe, Sekelwa Cosa, and Kokoette Bassey, Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds, Molecules. 2023 Jan; 28(2): 899.
[13] Ibrahim, H. (2015), Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Rad Res App Sci 8(3):265–275
[14] Mamdooh, N. W., & Naeem, G. A. (2022, July). The effect of temperature on green synthesis of silver nanoparticles. In AIP Conference Proceedings (Vol. 2450, No. 1). AIP Publishing.
[15]A. N. Al-Hakimi, T. M. Alresheedi, and R. A. Albarrak, “The effect of the Saudi haloxylon ammodendron shrub on silver nanoparticles: optimal biosynthesis, characterization, removability of mercury ions, antimicrobial and anticancer activities,” Inorganics, vol. 11, no. 6, pp. 246–316, 2023.
[16] K.S. Shaker, M.A. Muhi, M.Sh. Khalaf, H.L. Mansour,” Preparation of Silver Nanoparticles by Chemical Reaction Method at Different Reaction Temperatures and the Study of their Antibacterial Activity,“ Engineering and Technology Journal Vol. 35, Part B. No. 2, (2017).
[17] Singh, C., Kumar, J. Kumar, P., Chauhan, S. B., Tiwari, N. K., Mishra, S. K., Srikrishna, S. Saini, R., Nath, G. & Singh, J. 2019. Green synthesis of silver nanoparticles using aqueous leaf extract of Premna integrifolia (L.) rich in polyphenols and evaluation of their antioxidant, antibacterial and cytotoxic activity. Biotechnology & biotechnological equipment, 33(1), pp. 359-371.
[18] Jagtap, U. & Bapat, V. 2013. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crops Prod,. 46, pp. 132-137.

