Study of water temperature effect on the synthesis of gold nanoparticles by laser ablation technique
Ahmed O. Soary*1, Ali Saeed jassim2, and Adnan Hassoon Oraibi1
1Department of Physics, Faculty of Science, University of Kufa, Al-Najaf, Iraq
2Department of Geology, Faculty of Science, University of Kufa, Al-Najaf, Iraq
Corresponding author. E-mail: ahmed.alkillabi@uokufa.edu.iq*
DOI: https://doi.org/10.53796/hnsj62/12
Arabic Scientific Research Identifier: https://arsri.org/10000/62/12
Volume (6) Issue (2). Pages: 143 - 147
Received at: 2025-01-04 | Accepted at: 2025-01-15 | Published at: 2025-02-01
Abstract: In this study, gold nanoparticles were made by using a Q-switched Nd: YAG laser of 1064 nm wavelength. The process involved hitting a pure gold object in double distilled water with 90 laser pulses, each lasting 10 nanoseconds and using 700 mJ of energy. The experiments were conducted at different water temperatures. The study looked at how heating water affects the optical properties, surface shape, and size of nanoparticles. The results showed that as the water temperature rises, the particle size also increases. Additionally, when the water is heated to 50 degrees Celsius, the absorption spectra show a change towards red.
Keywords: switched Nd: YAG laser, , gold nanoparticles, water temperature, and PLAL.
- Introduction
Nanotechnology involves making, designing, and using very small materials called nanomaterials or nanostructures. It also includes knowing how the size of these materials affects their physical features. Nanotechnology focusses on very small structures or materials measured in nanometers, which are usually from less than one nanometer up to several hundred nanometers. The smaller the size of nanomaterials and the more concentrated they are, the more areas of benefit from them in several very important applications in science, technology, and other areas of our daily lives. material Properties depend on the chemical makeup and structure of the substances involved. Bulk materials are composed of many atoms, and they have energy ranges. These ranges are important because they affect the chemical and physical properties of solid materials. In nanomaterials, the electrical energy ranges of atoms become very small, which greatly affects most of the physical properties of materials. Nanoparticles have important properties such as electronic, optical, mechanical, electrical and chemical properties. These new properties of nanomaterials are very different from its properties when they were bulk materials. There are several types of methods for preparing nanoparticles such as Pulsed laser deposition PLD [4], photo reduction, chemical reduction [5], and electrochemical reduction [6]. Using laser to remove metal from a target placed in a vacuum cavity or in a certain gas or in air or in a liquid are important methods for producing nanomaterials and each of them has its own advantages. Using laser to remove metal from a target placed in a liquid such as water is an easy way to create metal nanoparticles. This method has gained a lot of interest. [7,8]. Using laser ablation in liquid allows for the easy creation of well-formed nanoparticles without needing additional heat processes, unlike other methods like laser ablation in vacuum and chemical vapour deposition. The producted metal nanoparticles by this method are as colloidal solution [11]. The goal of the study is investigating how water temperature affects the uptake and size of nanoparticles, as well as the concentration of the nanoparticles produced.
- Experimental
The quality of bulk gold plate used for production of gold NPS was (99.99%) and its thickness was 0.5 mm. By use Nd: YAG laser of wavelength 1064 nm wavelength, 6 Hz repeat rate and 10 ns pulse width. The gold object was positioned at the bottom of a 1 mL glass container filled with double-distilled water (DDW). The plate of gold was cleaned before the trial, and before each trial we cleaned the depth of the gold rod. The layer of double-distilled water above the target was 9 mm deep. Researchers looked at how gold nanoparticles behave in fluid by measuring how much light they absorb. Because nanoparticles grow quickly and clump together, all measures were taken right after they were created in water. Gold nanoparticles in distilled water appear pink and remain stable without settling. The size and form of gold nanoparticles were examined using AFM and SEM.
- Results and Discussion
Colloidal gold nanoparticles was created by using pulsed laser ablation of a plate of gold put in the quartz vessel that holding 1ml of DDW. The energy focused for each pulse was 700 mJ, with a total of 90 bursts using a 1064 nm Nd: YAG laser. At different water temperature (5,25,50 co).
Fig. 1 shows UV–VIS absorption bands of gold nanoparticles. The spectra have a noticeable peak at about 522nm. It was discovered that samples made at high water temperatures absorb less than those made at lower temperatures.
Figure 2 displays SEM pictures of gold nanoparticles created by using a laser on metal plates that were placed in deionized water. The laser has a wavelength of 1064 nm. 90 pulse and 700 mJ energy with different temperatures of water (50 ,25 and 5co respectively). at room temperature (5co) the smaller size particle was more concentration and at room temperature and at 5co the shape of particles was circular, but the particles shapes become random shapes when the water is heated to 50 co.
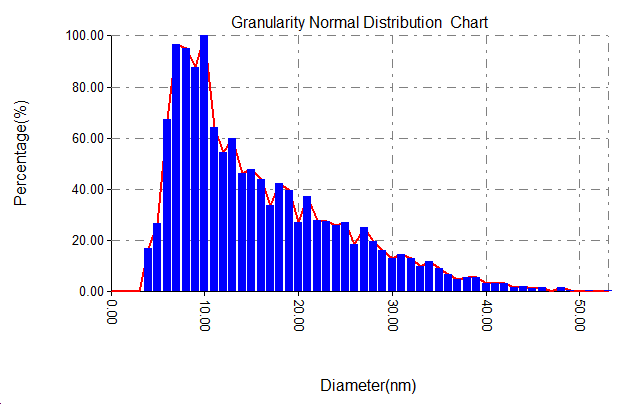
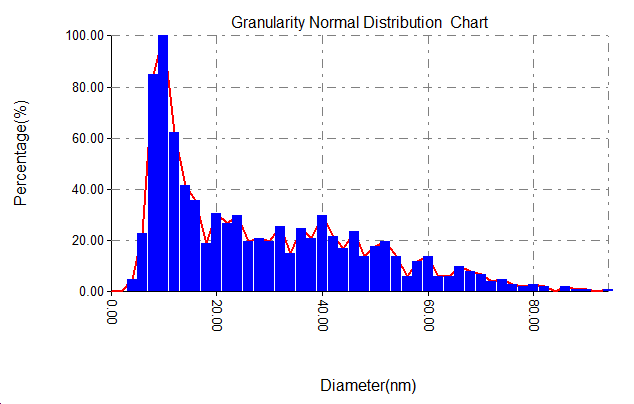
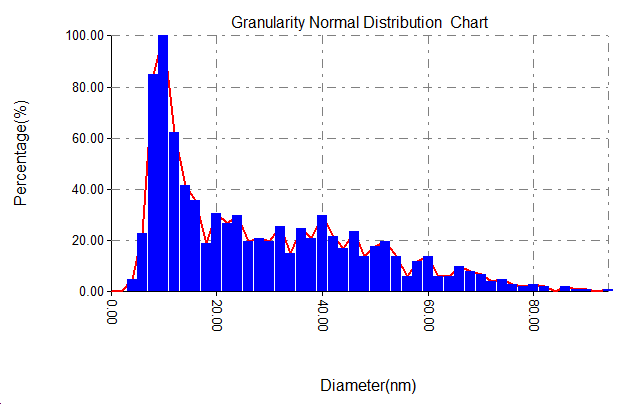
Figure 3 displays pictures from an AFM and the gold nanoparticles size ranges. With temperature (50, 25 and 5co respectively) and the 1064 nm laser wavelength, 700 mJ energy and 90 pulse, the nanoparticles made had average sizes of 15 nm and 27 nm. The growth of nanoparticle size is mainly affected by the cooling rate of the dissolved species in the liquid [10]. The growth of nanoparticles is mainly affected by the cooling rate of the dissolved species in the liquid. With the decrease in water temperature, we expected an increase in the cooling rate, and vice versa, an increase in the cooling rate will lead to the continuation of clustering, i.e. the production of larger particles. This means that the increase in water temperature caused an increase in the sizes of nanoparticles [11]. At 50 degrees Celsius, the size range was between 8 and 12 nanometers, and between 6 and 11 nanometers at room temperature.
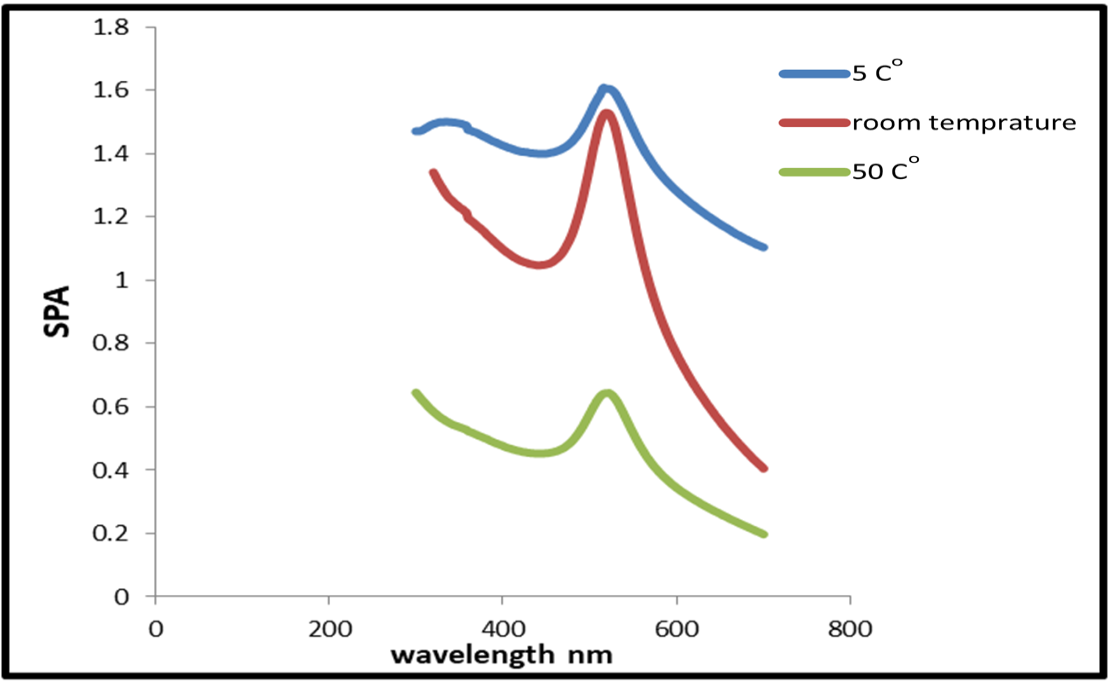
Figure 1: Spectra of Absorption of the plasmon band of gold nanoparticles, obtained by laser ablation at different water temperature.
Table 1: absorbance spectra at Minimum values as a function of water temperature.
|
Water temperature co |
Wavelength (nm) |
Abs. |
|
50 |
522 |
0.644 |
|
25 |
518 |
1.529 |
|
5 |
526 |
1.652 |

A

B

Figure 2: The SEM image of gold nanoparticles on silicon substrate prepared at different water temperature ((A)25 co, (B)50 co, (C)5 co)
A
B
C
Figure 3: AFM images and corresponding size distribution of gold nanoparticles prepared at (A)25, (B)50, (C)5
Conclusion
Using laser ablation technique in double-distilled water at different water temperatures, we were able to successfully synthesize gold nanoparticles. From this work, we concluded that as the water temperature increases, the size of the nanoparticles increases and their concentration decreases. The opposite happens as the water temperature decreases, its size decreases and its concentration increase. At higher water temperatures, the absorption peaks of the synthesized samples are smaller than those synthesized at lower temperatures of 5 °C.
Reference
[1] G. Cao, Nanostructures and Nanomaterials, Synthesis, Properties, and Applications, Copyright @ by Imperial College Press, p1, (2004).
[2] A. Pyatenko, K. Shimokawa, M. Yamaguchi, O. Nishi- mura and M. Suzuki, “Synthesis of Silver Nanoparticles by Laser Ablation in Pure Water,” Applied Physics A, Vol. 79, No. 4-6, 2004, pp. 803-806.
[3] N.V. Nikonorov,, A.I. Sidorov, and V.A. Tsekhomskii, “Silve Nanoparticles in Oxide Glasses:Technologies and Properties” David Pozo Perez , ISBN: 978-953-307-028-5 p.p. 177-201(2010)
[4] N. R. Michael, Ashfold, F. Claeyssens, G. M. Fuge and S. J. Henley (Pulsed laser ablation and deposition of thin films) C h e m . S o c . R e v . , 2 0 0 4 , 3 3 , 2 3 – 3 1
[5] C. Chen,Wu, X. Qiao, J. H. Wang, F. Tan,and S. Li (A novel chemical route to
prepare ZnO nanoparticles) Materials Letters 60, 1828–1832 (2006)..
[6] M .J. Haider, M. S. Mehdi(Effect of Experimental Parameters on the Fabrication of Silver Nanoparticles by Laser Ablation) Tech.Journal, Vol. 32,Part (B), No.4, (2014).
[7]H. Imam, K. A.Elsayed, L. Z. Ismail, M. Afify and M. Atta Khedr( Fabrication of Silver Nanoparticles by Laser Ablation in Liquid Solution) Life Science Journal;10(4).( 2013)
[8] W. M. Khilkala, G. A. Al-Dahash and S. N. Abdul Wahid (Preparation and Characterization of Cu nanoparticles by Laser Ablation in NaOH Aqueous Solution) International Journal of Current Engineering and Technology, Vol.4, No.4 (Aug 2014).
[9] C. Y. Liang, T. S. Shimizu,, and N. Koshizaki, Journal of J. Phys. Chem. B, Vol.107, pp.9220, (2003)
[10] A. HAHN, S. BARCIKOWSKI and B. N. CHICHKOV (Influences on Nanoparticle Production during Pulsed Laser Ablation) JLMN-Journal of Laser Micro/Nanoengineering Vol. 3, No. 2, (2008).
[11] J. A. Sylvestre, V. Kabashin, E. Sacher, M. Meunier,and J. T. Luong (Stabilization and Size Control of Gold Nanoparticles during Laser Ablation in Aqueous Cyclodextrins) J.AM. CHEM. SOC. 126, 7176-7177 (2004).
