Green Methods for Gold Nanoparticle Synthesis: Properties, Characterization, and Diverse Applications – Review Article
الطرق الخضراء لتخليق جسيمات الذهب النانوية الخصائص والتوصيف والتطبيقات – مقالة مراجعة
Laith Saleh Alhiti1*, Estabraq Hamid Hashim2, Fatima Haider Abdullah3
1*Medical physics Department, College of Applied Sciences-Heet, University of Anbar, Iraq.
2Biophysics Department, College of Applied Sciences-Heet, University of Anbar, Iraq.
3Department of Laser Physics, College of Science for Girls, University of Babyl
*Corresponding Author: laith2011alhiti@uoanbar.edu.iq
DOI: https://doi.org/10.53796/hnsj63/12
Arabic Scientific Research Identifier: https://arsri.org/10000/63/12
Pages: 249 - 265
Received at: 2025-02-07 | Accepted at: 2025-02-15 | Published at: 2025-03-01
Abstract: This paper presents a review on the environmentally friendly methods for the synthesis of gold nanoparticles. It explains the green synthesis methods, particle properties, and potential biological applications. This study demonstrates that gold nanoparticles have remarkable light absorption, good chemical stability, and multiple interactions with biological systems. Since conventionally synthesized nanoparticles are equipped with toxic chemicals, alternative, safer methods must be discovered. The paper discusses the synthesis of gold nanoparticles through different “green” methods, using natural sources such as plants and microorganisms—fungi and bacteria—as reducing and catalyzing agents.These green methods generally result in the production of nanoparticles with high purity to avoid contamination, long-term stability to ensure durability, and low toxicity for biomedical safety. Technically, the paper discusses the characterization techniques of different produced nanoparticles by UV–Vis spectroscopy and scanning electron microscopy. The research results show that the particle properties, such as size, dimensions, morphology, distribution, and crystallinity, are essential features that directly affect the performance of the particles under actual working conditions for most applications. This research proves the importance, feasibility, and usefulness of further research on the synthesis methods of gold nanoparticles. Expanding their application areas, especially in the future biotechnology and medical engineering sectors, is very necessary.
Keywords: Green Synthesis, Gold Nanoparticles (AuNPs), Biosynthesis, Nanotechnology, Biomedical Applications.
المستخلص: تقدم هذه الورقة مراجعة للطرق الصديقة للبيئة لتخليق جسيمات النانو الذهبية. وتشرح طرق التخليق الخضراء وخصائص الجسيمات والتطبيقات البيولوجية المحتملة. وتوضح هذه الدراسة أن جسيمات النانو الذهبية تتمتع بامتصاص ضوئي رائع واستقرار كيميائي جيد وتفاعلات متعددة مع الأنظمة البيولوجية. ونظرًا لأن الجسيمات النانوية المصنعة تقليديًا مزودة بمواد كيميائية سامة، فيجب اكتشاف طرق بديلة أكثر أمانًا. وتناقش الورقة تخليق جسيمات النانو الذهبية من خلال طرق "خضراء" مختلفة، باستخدام مصادر طبيعية مثل النباتات والكائنات الحية الدقيقة - الفطريات والبكتيريا - كعوامل اختزال وتحفيز. وتؤدي هذه الطرق الخضراء عمومًا إلى إنتاج جسيمات نانوية عالية النقاء لتجنب التلوث، واستقرار طويل الأمد لضمان المتانة، وسمية منخفضة للسلامة الطبية الحيوية. من الناحية الفنية، تناقش الورقة تقنيات توصيف الجسيمات النانوية المختلفة المنتجة بواسطة مطيافية الأشعة فوق البنفسجية والمرئية والمجهر الإلكتروني الماسح. وتظهر نتائج البحث أن خصائص الجسيمات، مثل الحجم والأبعاد والشكل والتوزيع والبلورية، هي سمات أساسية تؤثر بشكل مباشر على أداء الجسيمات في ظل ظروف العمل الفعلية لمعظم التطبيقات. ويثبت هذا البحث أهمية وجدوى وفائدة إجراء المزيد من البحوث حول طرق تخليق جسيمات النانو الذهبية. وتوسيع مجالات تطبيقها، وخاصة في قطاعات التكنولوجيا الحيوية والهندسة الطبية في المستقبل، أمر ضروري للغاية.
الكلمات المفتاحية: التخليق الأخضر، جسيمات الذهب النانوية (AuNPs)، التخليق الحيوي، تكنولوجيا النانو، التطبيقات الطبية الحيوية.
- Introduction
Nanoparticles have a long history in medicine since in ancient times they were used for therapeutic purposes such as colloidal gold. Modern applications of nanoparticles, however, did attract serious attention until the late 20th century when nanotechnology was relatively well advanced [1]. They are now extensively used for specific drug delivery systems, imaging and diagnostics, and cancer therapy. In most cases, nanoparticles are used to increase the accuracy of treatments due to enhanced solubility, stability, and focus of actions as well as side effects. Indeed, their applications cut across oncology, regenerative medicine, and vaccine development [2]. Nanoparticles have several physical and chemical properties because of their much larger surface area. Among several nanoparticles, metal nanoparticles of gold, silver, platinum, and palladium have gained prominent attention because of their catalytic activities, biological activities, optical activities, electrochemical applications, biotechnology, and electronic properties [3]. Further, they have plenty of applications in the field of analytical and electroanalytical fields since their properties are quite different compared with other metallic nanoparticles [4]. Apart from these, interest in AuNPs is quite strong for chemical stability and size with electronic structure-related electronic, optical, and spectroscopic properties used in research work. Moreover, the activities of AuNPs are also reported against animal and food pathogens [5]. The applications of AuNPs continue to broaden in studies related to pharmacokinetics, the environment, the space industry, as well as the analytical sector, which encompasses separation science. The stationary phases in separation techniques are ever-evolving to meet the regular demand in the field of the analytical sector [6].
- Green Synthesis of Gold Nanoparticles
An elementary scientific consideration is the synthesis of nanoscale gold in the controlled phase or morphologies. The preparation of colloidal gold was described by Michael Faraday almost 150 years back using phosphorous for the reduction of AuCl4 ions [17]. Over the last few years, several methodologies based on biological, physical, or chemical approaches have been developed for the synthesis of GNPs used in the fields of electrical, biotechnological, industrial, pharmaceutical, agricultural, or medical sectors. This method is used for the synthesis of well-defined compositions of gold nanostructures as colloids, clusters, wires, powders, tubes, rods, and thin films [18]. Physical and chemical approaches used for the synthesis of GNPs have been illustrated previously according to Figure 1. A few limitations were noticed in these synthesis approaches despite extensive research: they involved the use of harsh chemicals, highly stringent synthesis conditions, and high energy or capital requirements with less productivity [19]. The as-synthesized mix-shaped NPs need high-cost purification processes, such as the usage of a method of differential centrifugation. Further, these also led to more sludge and hazardous environmental risks due to the harmful solvents or additives [20]. Due to this, there has been an increased need for clean, nontoxic, ecologically friendly long-term synthesis methods. The major challenge is in developing high-yield, low-cost NPs production technologies. NPs have, in fact, attracted a wide variety of applications because of application diversity [21].
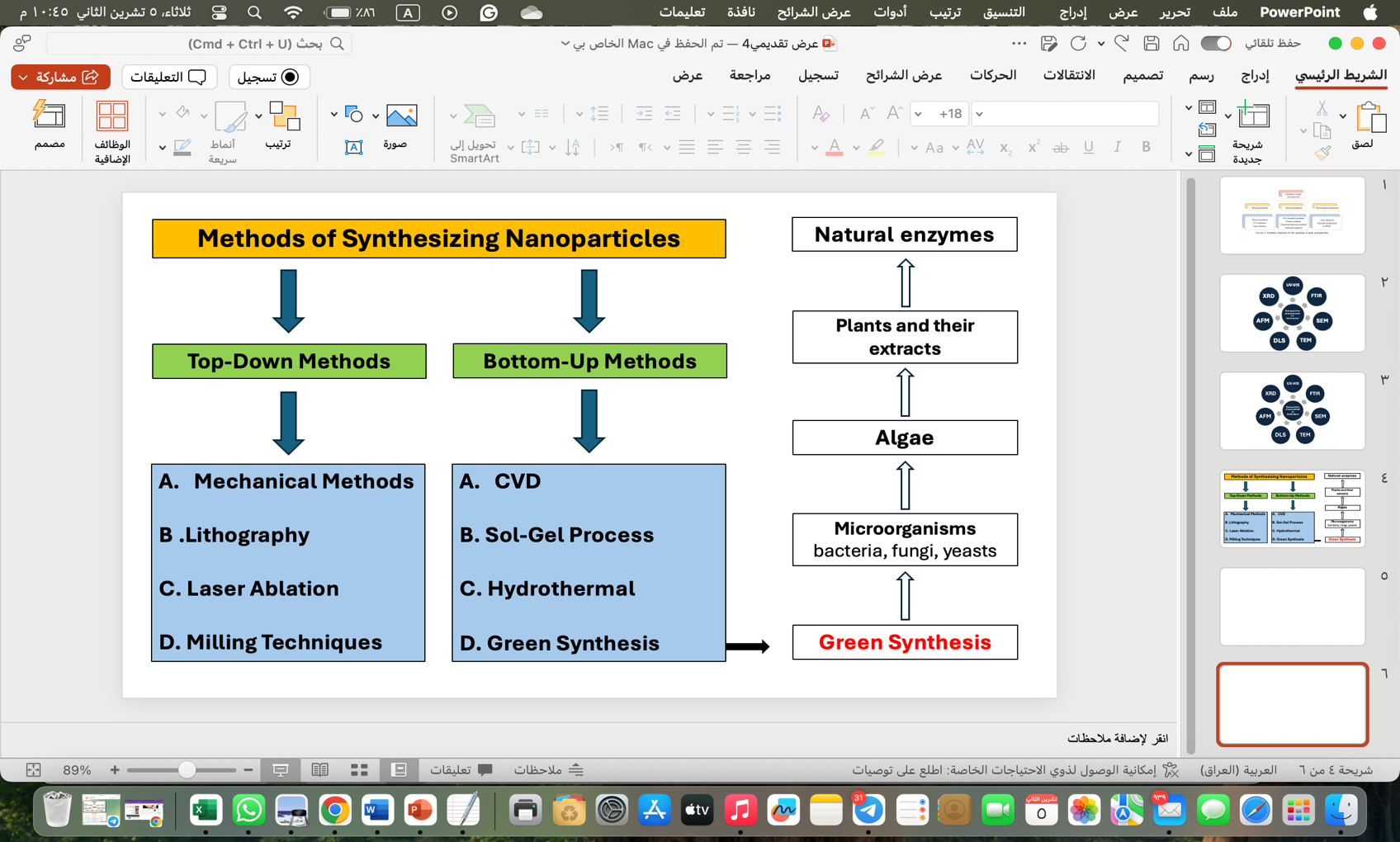
Figure 1: Schematic showing the methods for the synthesis of gold nanoparticles (AuNPs) [22].
-
- Plant-mediated synthesis
This is a process that depends on the natural compound of phenols and flavonoids found in the plants, which reduce the gold ions with no hazard or complexity of chemicals and thus need to be reduced to their nanometer size [23]. A solution of gold ions has to be brought into contact with the plant extract. It then catalyzes the reaction reduction, and nanoparticles are formed. This method is very simple and environmentally friendly because it uses plant resources that are abundant and easily accessible and does not require much energy or hazardous chemicals [24]. The particles obtained can be applied to medical fields such as drug carriers as well as in biosensor technology, hence a very promising and effective way of nanomaterial production [25].
Table 1: Biosynthesis of gold nanoparticles using different plant-reducing agents (leaves, fruits, flowers, seeds, bark, oils).
|
No. |
Plant species |
Part used |
Shape |
Analytical Techniques |
Size (nm) |
Applications |
Ref. |
|
C. auriculata |
Leaves |
|
|
15 – 25 |
|
[27] |
|
|
D. kotschyi |
Leaves |
|
|
11 |
|
[28] |
|
|
C. papaya |
Leaves |
|
|
2 – 20 |
|
[29] |
|
|
N. oleander |
Leaves |
|
|
2 – 10 |
|
[30] |
|
|
P. dactylifera |
Leaves |
|
|
32 – 45 |
|
[31] |
|
|
P. benghalensis |
Leaves |
|
|
13.07 |
|
[32] |
|
|
Z. mauritiana |
Leaves |
|
|
20 – 40 |
|
[33] |
|
|
S. nigrum |
Leaves |
|
|
50 |
|
[34] |
|
|
V. negundo |
Leaves |
|
|
98.65 – 71.86 |
|
[35] |
|
|
T. decandra |
Leaves |
|
|
37.7 – 79.9 |
|
[36] |
-
- Bacterial synthesis
Synthesis of gold nanoparticles using bacteria, this process involves the reduction of gold ions by bacteria through completely biological chemical reactions and the formation of gold nanoparticles. Because the bacteria secrete enzymes or some kind of natural compounds that react with the gold ions [77]. This method is described as environmentally friendly because it avoids the use of chemicals, and it is also economical. The size and geometric shape of the nanoparticles can be controlled by modifying the environmental conditions of the synthesis. [78].
Table 2: Biosynthesis of gold nanoparticles using different bacterial reducing agents.
|
No. |
Scientific name |
Category |
Shape |
Analytical Techniques |
Size (nm) |
Applications |
Ref. |
|
Streptomyces sp. |
Bacteria |
Spherical |
|
40 |
|
[79] |
|
|
Brevibacterium casei |
Bacteria |
Spherical |
|
10 – 50 |
|
[80] |
|
|
Geobacillus stearothermophilus |
Bacteria |
Spherical |
|
12 – 14 |
|
[81] |
|
|
Bacillus stearothermophilus |
Bacteria |
Spherical, triangular |
|
5 – 30 |
|
[82] |
|
|
Shewanella oneidensis |
Bacteria |
Spherical |
|
2 – 50 |
|
[83] |
|
|
staphylococcus epidermidis |
Bacteria |
Spherical |
|
20 – 25 |
|
[84] |
|
|
Lactobacillus sp. |
Bacteria |
Hexagonal |
|
60 – 30 |
|
[85] |
|
|
pseudomonas veronii |
Bacteria |
Variable |
|
5 – 25 |
|
[86] |
|
|
Paracoccus haeundaensis BC74171 T |
Bacteria |
Spherical |
|
3.46±20.93 |
|
[87] |
|
|
Bacillus cereus |
Bacteria |
Spherical |
|
10 – 30 |
|
[88] |
-
- Fungal synthesis
Fungi-mediated synthesis involves the ability of the fungi to secrete enzymes, which further reduce the gold ions into nanoparticles. This acts as a completely natural process without the enforcement of any sort of harmful chemicals, with fungi acting as biocatalysts [89]. The methodology is user-friendly and productive, whereby the nanoparticles thus formed find a great lot of applications in the field of medicinal and biotechnological purposes [90].
Table 3: Biosynthesis of gold nanoparticles using different fungal reducing agents.
|
No. |
Scientific name |
Category |
Shape |
Analytical Techniques |
Size (nm) |
Applications |
Ref. |
|
Trichoderma viride and Hypocrea lixii |
Fungi |
Spherical, irregular |
|
20 – 30 |
|
[91] |
|
|
Volvariella volvacea |
Fungi |
Triangular, spherical, hexagonal |
|
20 – 150 |
|
[92] |
|
|
Inonotus obliquus |
Fungi |
Spherical, triangle, hexagonal |
|
23 |
|
[93] |
|
|
Candida albicans |
Fungi |
Spherical |
|
20 – 40 |
|
[94] |
|
|
Helminthosporium solani |
Fungi |
Variable |
|
3 – 80 |
|
[95] |
|
|
Colletotrichum sp. |
Fungi |
Decahedral |
|
30 – 50 |
|
[96] |
|
|
Neurospora crassa |
Fungi |
Spherical |
|
3 – 80 |
|
[97] |
|
|
Penicillium brevicompactum |
Fungi |
Spherical |
|
20 – 60 |
|
[98] |
|
|
Phanerochaete chrysosporium |
Fungi |
Spherical |
|
20 – 110 |
|
[99] |
|
|
Cylindrocladium floridanum |
Fungi |
Spherical |
|
19.05 |
|
[100] |
-
- Algal synthesis
The biosynthesis of gold nanoparticles is generally referred to as the reduction of gold ions to nanoparticles by algae using their natural biological property [101]. The choice of the algae species, extraction of biologically active molecules like proteins and sugars, which assist in this reduction, immobilization of the nanoparticles into aqueous extracts, and finally their stabilization into an ionic state is carried out in this process [102].
Table 4: Biosynthesis of gold nanoparticles using different algal reducing agents.
|
No. |
Scientific name |
Category |
Shape |
Analytical Techniques |
Size (nm) |
Applications |
Ref. |
||||||
|
Sargassum muticum |
Algae |
Spherical |
|
1.18 ±5.42 |
|
[105] |
|||||||
|
Prasiola crispa |
Algae |
Spherical |
|
9.8 |
|
[106] |
|||||||
|
Stylidium tenerrimum |
Algae |
Anisotropic |
|
5 – 45 |
|
[107] |
|||||||
|
Stoechospermum marginatum |
Algae |
Spherical |
|
18.7 – 93.7 |
|
[108] |
|||||||
|
Sargassum swartzii |
Algae |
Spherical |
|
35 |
|
[109] |
|||||||
|
Galaxaura Elongate |
Algae |
Spherical |
|
3.85 – 77.13 |
|
[110] |
|||||||
|
Prasiola crispa |
Algae |
Spherical |
|
5 – 25 |
|
[111] |
|||||||
|
Padina gymnospora |
Algae |
Spherical |
|
53 – 67 |
|
[112] |
|||||||
|
Lemanea fluviatilis (L.) C.Ag. |
Algae |
face-centered cubic |
|
5.9 |
|
[113] |
|||||||
|
Kappaphycus alvarezii |
Algae |
Spherical |
|
10 – 40 |
|
[114] |
|||||||
- Gold Nanoparticles Characterization Techniques
Characterization of block stabilized gold nanoparticle samples by various techniques is expected to lead to homogeneity and orientation of the metal particles [115]. These techniques include surface characterization microscopy, spectrophotometry, X-ray spectroscopy, and flux properties.
-
- UV-Visible Spectroscopy (UV-Vis (
The Ultraviolet-Visible (UV-Vis) spectroscopy technique is used to study the core chemistries of gold nanoparticles (AuNPs) via their interaction and absorption of UV and visible light rays. This can be explained by Gold Nanoparticles because AuNPs show what is known as Localized Surface Plasmon Resonance (LSPR), where there is vigorous oscillation of electrons on the surface of the nanoparticle upon interaction with incoming light [116]. Hence, there is a very intense peak of absorption noticed [117].
-
- Fourier Transform Infrared Spectroscopy (FTIR (
Fourier Transform Infrared (FTIR) spectroscopy determines chemical composition and surface functional groups of AuNPs; therefore, it is a valuable technique to know how molecules or functional groups are attached to the surface of the nanoparticles. The Fourier-transform infrared spectrometer measures the absorption of infrared light by the molecules at different wavelengths; the absorbed light excites vibration (stretching or bending) of the bonds between atoms in a molecule. The laboratory collects the vibrational spectra as peaks on an infrared spectrum to give a “fingerprint” of the chemical bonds and functional groups present [120].
-
- X-ray diffraction (XRD) analysis
X-ray diffraction is one of the important procedures that can be used to study the structural features of gold nanoparticles synthesized by environmentally friendly methods. The crystalline structure of the particles is viewed using this technique by recording the angles of diffraction from the sample with X-rays incident upon it. X-rays are directed onto a sample of gold nanoparticles, after which angles at which the rays are reflected off and scattered by the atoms constituting the particles are measured; in the end, peaks appear on the spectrum, with each corresponding to a set of crystalline grains [122].
-
- Transmission Electron Microscopy (TEM (
TEM is an advanced method of study for the structural and optical properties of green-synthesized gold nanoparticles [125]. An accurate view of the particles at the nanoscale can be obtained by TEM; hence, the shape, size, and distribution may be understood in detail. In TEM, the sample is bombarded by a beam of electrons if it is scanned with electrons penetrating the particles, generating an image based on how these electrons interact with the atoms inside the particles. Such images give the exact dimensions of the particles, thus helping in understanding size and shape distributions. The TEM will also show the crystal structure of the particles that way if the particles are of regular or random crystal structure [126].
-
- Atomic Force Microscopy (AFM (
In this view, atomic force microscopy (AFM) ascertains the structural properties of gold nanoparticles in a diversity of applications. The exquisiteness of three-dimensional images of nanoparticles for size, shape, and surface distribution made possible by AFM is of much value [128]. Since the chemical and physical properties of gold nanoparticles are hugely dependent on their surface properties and size, size control became crucial and a study of its application in various fields [129]
- Discussion
The size values of gold nanoparticles in Table 1 indicate that there is a large variation in the particle sizes, indicating that the type of plant extract is responsible for the synthesis of particles with different sizes, perhaps due to some chemical and biological factors present in the plant extract, which may induce the size of the nanoparticles. Therefore, it is necessary to choose the appropriate plant extract to synthesize the desired nanoparticles. Similarly, for the nanoparticles synthesized using fungal or bacterial extracts in Tables 2, 3 and 4. For the nanoparticles synthesized using bacterial extract, a relative stability was observed among most of the nanoparticle size distributions, with the majority of particles being between 10 and 40 nm. The more uniform distribution here compared to the plant extract indicates the fact that the bacterial extract can provide a more controlled environment for the production of nanoparticles with similar sizes. For the plant extract, a large variation in the sizes of the nanoparticles should be observed, with some particles being very large while others being small in size. This difference can be attributed to the various phytochemicals and enzymes; hence, nanoparticles of different sizes are generated, depending on the effects of these compounds on particle growth and synthesis.
Hence, the disparity in the size of the nanoparticles synthesized by each extract is due to the chemical and biological composition present in each organism, that is, fungi and algae. Different organic compounds and enzymes are contained in those two respective organisms, which then influence the speed or rather rate of nanoparticle formation and their sizes in different ways.
- Conclusions
The present work deals with environmentally acceptable protocols available for the synthesis of gold nanoparticles, and it deals with their properties, characterization techniques, and applications. The green methods of synthesis consider non-toxic and natural materials rather than utilizing hazardous chemical reagents that cause severe effects on the environment. Several methods for the synthesis of gold nanoparticles using plants and microorganisms, bacteria, and fungi are established in this paper as safer and more economical substitutes. The methods produce nanoparticles of required physical and chemical properties, which are as effective as the traditional ones. Antioxidant and antibacterial properties in gold nanoparticles produced through green synthesis suggest their potential application in newer fields of biomedicine for the fabrication of biosensors, targeted therapies for the treatment of cancer by attacking cancer cells, and drug delivery systems. In addition, the techniques employed in the characterization of nanoparticles using electron microscopy and spectroscopy are reviewed. This helps in the study of structure and properties. The paper concludes by underlining the need for environmentally friendly techniques in nanomaterial synthesis and encouraging their extension to reduce effects on the environment and increase sustainability, with further research into uses in medicine, industry, and biotechnology.
- References
[1] A. Balfourier, J. Kolosnjaj-Tabi, N. Luciani, F. Carn, and F. Gazeau, “Gold-based therapy: From past to present,” Proceedings of the National Academy of Sciences, vol. 117, no. 37, pp. 22639–22648, Sep. 2020, doi: 10.1073/pnas.2007285117.
[2] F.-Y. Kong, J.-W. Zhang, R.-F. Li, Z.-X. Wang, W.-J. Wang, and W. Wang, “Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications,” Molecules, vol. 22, no. 9, p. 1445, Aug. 2017, doi: 10.3390/molecules22091445.
[3] G. L. Hornyak and A. K. Rao, “Fundamentals of Nanoscience (and Nanotechnology),” in Nanoscience in Dermatology, Elsevier, 2016, pp. 15–29. doi: 10.1016/B978-0-12-802926-8.00002-1.
[4] M. Sajid and J. Płotka-Wasylka, “Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences,” Microchemical Journal, vol. 154, p. 104623, May 2020, doi: 10.1016/j.microc.2020.104623.
[5] V. Amendola, R. Pilot, M. Frasconi, O. M. Maragò, and M. A. Iatì, “Surface plasmon resonance in gold nanoparticles: a review,” Journal of Physics: Condensed Matter, vol. 29, no. 20, p. 203002, May 2017, doi: 10.1088/1361-648X/aa60f3.
[6] J. Jeevanandam, A. Barhoum, Y. S. Chan, A. Dufresne, and M. K. Danquah, “Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations,” Beilstein Journal of Nanotechnology, vol. 9, pp. 1050–1074, Apr. 2018, doi: 10.3762/bjnano.9.98.
[7] S. Menon, R. S., and V. K. S., “A review on biogenic synthesis of gold nanoparticles, characterization, and its applications,” Resource-Efficient Technologies, vol. 3, no. 4, pp. 516–527, Dec. 2017, doi: 10.1016/j.reffit.2017.08.002.
[8] S. Ahmed, Annu, S. Ikram, and S. Yudha S., “Biosynthesis of gold nanoparticles: A green approach,” J Photochem Photobiol B, vol. 161, pp. 141–153, Aug. 2016, doi: 10.1016/j.jphotobiol.2016.04.034.
[9] A. I. Usman, A. Abdul Aziz, and O. Abu Noqta, “APPLICATION OF GREEN SYNTHESIS OF GOLD NANOPARTICLES: A REVIEW,” J Teknol, vol. 81, no. 1, Dec. 2018, doi: 10.11113/jt.v81.11409.
[10] I. Khan, K. Saeed, and I. Khan, “Nanoparticles: Properties, applications and toxicities,” Arabian Journal of Chemistry, vol. 12, no. 7, pp. 908–931, Nov. 2019, doi: 10.1016/j.arabjc.2017.05.011.
[11] J. Dolai, K. Mandal, and N. R. Jana, “Nanoparticle Size Effects in Biomedical Applications,” ACS Appl Nano Mater, vol. 4, no. 7, pp. 6471–6496, Jul. 2021, doi: 10.1021/acsanm.1c00987.
[12] P. Singh, S. Pandit, V. R. S. S. Mokkapati, A. Garg, V. Ravikumar, and I. Mijakovic, “Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer,” Int J Mol Sci, vol. 19, no. 7, p. 1979, Jul. 2018, doi: 10.3390/ijms19071979.
[13] J. Ma, S. M.-Y. Lee, C. Yi, and C.-W. Li, “Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications – a review,” Lab Chip, vol. 17, no. 2, pp. 209–226, 2017, doi: 10.1039/C6LC01049K.
[14] N. Li, P. Zhao, and D. Astruc, “Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity,” Angewandte Chemie International Edition, vol. 53, no. 7, pp. 1756–1789, Feb. 2014, doi: 10.1002/anie.201300441.
[15] J. B. Vines, J.-H. Yoon, N.-E. Ryu, D.-J. Lim, and H. Park, “Gold Nanoparticles for Photothermal Cancer Therapy,” Front Chem, vol. 7, Apr. 2019, doi: 10.3389/fchem.2019.00167.
[16] B. De Berardis, M. Marchetti, A. Risuglia, F. Ietto, C. Fanizza, and F. Superti, “Exposure to airborne gold nanoparticles: a review of current toxicological data on the respiratory tract,” Journal of Nanoparticle Research, vol. 22, no. 8, p. 235, Aug. 2020, doi: 10.1007/s11051-020-04966-9.
[17] H. Hassan, P. Sharma, Mohd. R. Hasan, S. Singh, D. Thakur, and J. Narang, “Gold nanomaterials – The golden approach from synthesis to applications,” Mater Sci Energy Technol, vol. 5, pp. 375–390, 2022, doi: 10.1016/j.mset.2022.09.004.
[18] S. светлана Tsekhmistrenko et al., “BIONANOTECHNOLOGIES: SYNTHESIS OF METALS’ NANOPARTICLES WITH USING PLANTS AND THEIR APPLICATIONS IN THE FOOD INDUSTRY: A REVIEW,” Journal of microbiology, biotechnology and food sciences, vol. 10, no. 6, p. e1513, Jun. 2021, doi: 10.15414/jmbfs.1513.
[19] M. I. Anik, N. Mahmud, A. Al Masud, and M. Hasan, “Gold nanoparticles (GNPs) in biomedical and clinical applications: A review,” Nano Select, vol. 3, no. 4, pp. 792–828, Apr. 2022, doi: 10.1002/nano.202100255.
[20] P. G. Jamkhande, N. W. Ghule, A. H. Bamer, and M. G. Kalaskar, “Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications,” J Drug Deliv Sci Technol, vol. 53, p. 101174, Oct. 2019, doi: 10.1016/j.jddst.2019.101174.
[21] A. Gour and N. K. Jain, “Advances in green synthesis of nanoparticles,” Artif Cells Nanomed Biotechnol, vol. 47, no. 1, pp. 844–851, Dec. 2019, doi: 10.1080/21691401.2019.1577878.
[22] I. Hammami, N. M. Alabdallah, A. Al jomaa, and M. kamoun, “Gold nanoparticles: Synthesis properties and applications,” J King Saud Univ Sci, vol. 33, no. 7, p. 101560, Oct. 2021, doi: 10.1016/j.jksus.2021.101560.
[23] M. Hassanisaadi, G. H. S. Bonjar, A. Rahdar, S. Pandey, A. Hosseinipour, and R. Abdolshahi, “Environmentally Safe Biosynthesis of Gold Nanoparticles Using Plant Water Extracts,” Nanomaterials, vol. 11, no. 8, p. 2033, Aug. 2021, doi: 10.3390/nano11082033.
[24] K. K. Bharadwaj et al., “Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics,” Molecules, vol. 26, no. 21, p. 6389, Oct. 2021, doi: 10.3390/molecules26216389.
[25] A. Karnwal et al., “Gold Nanoparticles in Nanobiotechnology: From Synthesis to Biosensing Applications,” ACS Omega, vol. 9, no. 28, pp. 29966–29982, Jul. 2024, doi: 10.1021/acsomega.3c10352.
[26] H. Chandran, G. S. Ramakrishnan, J. R. Mekala, and S. R. Anjaneyulu, “Evaluating the Synergistic Antioxidant, Anti-microbial and Adsorbent Potential of Andrographis Paniculata Extract and Gold Nanoparticles,” Cell Biochem Biophys, Dec. 2024, doi: 10.1007/s12013-024-01627-9.
[27] V. Ganesh Kumar et al., “Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata,” Colloids Surf B Biointerfaces, vol. 87, no. 1, pp. 159–163, Oct. 2011, doi: 10.1016/j.colsurfb.2011.05.016.
[28] N. Dorosti and F. Jamshidi, “Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: Synthesis, characterization, and evaluation of anticancer and antibacterial activity,” J Appl Biomed, vol. 14, no. 3, pp. 235–245, Aug. 2016, doi: 10.1016/j.jab.2016.03.001.
[29] T. Muthukumar, Sudhakumari, B. Sambandam, A. Aravinthan, T. P. Sastry, and J.-H. Kim, “Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects,” Process Biochemistry, vol. 51, no. 3, pp. 384–391, Mar. 2016, doi: 10.1016/j.procbio.2015.12.017.
[30] K. Tahir et al., “Nerium oleander leaves extract mediated synthesis of gold nanoparticles and its antioxidant activity,” Mater Lett, vol. 156, pp. 198–201, Oct. 2015, doi: 10.1016/j.matlet.2015.05.062.
[31] M. F. Zayed and W. H. Eisa, “Phoenix dactylifera L. leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 121, pp. 238–244, Mar. 2014, doi: 10.1016/j.saa.2013.10.092.
[32] B. Paul, B. Bhuyan, D. Dhar Purkayastha, M. Dey, and S. S. Dhar, “Green synthesis of gold nanoparticles using Pogestemon benghalensis (B) O. Ktz. leaf extract and studies of their photocatalytic activity in degradation of methylene blue,” Mater Lett, vol. 148, pp. 37–40, Jun. 2015, doi: 10.1016/j.matlet.2015.02.054.
[33] B. Sadeghi, “Zizyphus mauritiana extract-mediated green and rapid synthesis of gold nanoparticles and its antibacterial activity,” J Nanostructure Chem, vol. 5, no. 3, pp. 265–273, Sep. 2015, doi: 10.1007/s40097-015-0157-y.
[34] A. Muthuvel, K. Adavallan, K. Balamurugan, and N. Krishnakumar, “Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties,” Biomedicine & Preventive Nutrition, vol. 4, no. 2, pp. 325–332, Apr. 2014, doi: 10.1016/j.bionut.2014.03.004.
[35] P. Renuga Devi, C. Senthil Kumar, P. Selvamani, N. Subramanian, and K. Ruckmani, “Synthesis and characterization of Arabic gum capped gold nanoparticles for tumor-targeted drug delivery,” Mater Lett, vol. 139, pp. 241–244, Jan. 2015, doi: 10.1016/j.matlet.2014.10.010.
[36] G. R. and S. D.V.L., “Characterization and antimicrobial activity of gold and silver nanoparticles synthesized using saponin isolated from Trianthema decandra L.,” Ind Crops Prod, vol. 51, pp. 107–115, Nov. 2013, doi: 10.1016/j.indcrop.2013.08.055.
[37] K. Gopinath et al., “Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities,” Microb Pathog, vol. 101, pp. 1–11, Dec. 2016, doi: 10.1016/j.micpath.2016.10.011.
[38] Y.-J. Kim et al., “Rapid green synthesis of silver and gold nanoparticles using<em> Dendropanax morbifera</em> leaf extract and their anticancer activities,” Int J Nanomedicine, vol. Volume 11, pp. 3691–3701, Aug. 2016, doi: 10.2147/IJN.S97181.
[39] A. Rajan, V. Vilas, and D. Philip, “Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles,” J Mol Liq, vol. 212, pp. 331–339, Dec. 2015, doi: 10.1016/j.molliq.2015.09.013.
[40] M. R. Bindhu and M. Umadevi, “Antibacterial activities of green synthesized gold nanoparticles,” Mater Lett, vol. 120, pp. 122–125, Apr. 2014, doi: 10.1016/j.matlet.2014.01.108.
[41] R. Geetha, T. Ashokkumar, S. Tamilselvan, K. Govindaraju, M. Sadiq, and G. Singaravelu, “Green synthesis of gold nanoparticles and their anticancer activity,” Cancer Nanotechnol, vol. 4, no. 4–5, pp. 91–98, Aug. 2013, doi: 10.1007/s12645-013-0040-9.
[42] N. Basavegowda, A. Idhayadhulla, and Y. R. Lee, “Phyto-synthesis of gold nanoparticles using fruit extract of Hovenia dulcis and their biological activities,” Ind Crops Prod, vol. 52, pp. 745–751, Jan. 2014, doi: 10.1016/j.indcrop.2013.12.006.
[43] S. Lokina, R. Suresh, K. Giribabu, A. Stephen, R. Lakshmi Sundaram, and V. Narayanan, “Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 129, pp. 484–490, Aug. 2014, doi: 10.1016/j.saa.2014.03.100.
[44] M. R. Bindhu and M. Umadevi, “Silver and gold nanoparticles for sensor and antibacterial applications,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 128, pp. 37–45, Jul. 2014, doi: 10.1016/j.saa.2014.02.119.
[45] A. K. Mittal, J. Bhaumik, S. Kumar, and U. C. Banerjee, “Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential,” J Colloid Interface Sci, vol. 415, pp. 39–47, Feb. 2014, doi: 10.1016/j.jcis.2013.10.018.
[46] M. Ganeshkumar, M. Sathishkumar, T. Ponrasu, M. G. Dinesh, and L. Suguna, “Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery,” Colloids Surf B Biointerfaces, vol. 106, pp. 208–216, Jun. 2013, doi: 10.1016/j.colsurfb.2013.01.035.
[47] N. Basavegowda, A. Idhayadhulla, and Y. R. Lee, “Phyto-synthesis of gold nanoparticles using fruit extract of Hovenia dulcis and their biological activities,” Ind Crops Prod, vol. 52, pp. 745–751, Jan. 2014, doi: 10.1016/j.indcrop.2013.12.006.
[48] CH. Ramamurthy et al., “The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties,” Colloids Surf B Biointerfaces, vol. 102, pp. 808–815, Feb. 2013, doi: 10.1016/j.colsurfb.2012.09.025.
[49] RM. Ganesan and H. Gurumallesh Prabu, “Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications,” Arabian Journal of Chemistry, vol. 12, no. 8, pp. 2166–2174, Dec. 2019, doi: 10.1016/j.arabjc.2014.12.017.
[50] S. Naraginti and A. Sivakumar, “Eco-friendly synthesis of silver and gold nanoparticles with enhanced bactericidal activity and study of silver catalyzed reduction of 4-nitrophenol,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 128, pp. 357–362, Jul. 2014, doi: 10.1016/j.saa.2014.02.083.
[51] T. V. M. Sreekanth, P. C. Nagajyothi, N. Supraja, and T. N. V. K. V. Prasad, “Evaluation of the antimicrobial activity and cytotoxicity of phytogenic gold nanoparticles,” Appl Nanosci, vol. 5, no. 5, pp. 595–602, Jun. 2015, doi: 10.1007/s13204-014-0354-x.
[52] M. M. Poojary, P. Passamonti, and A. V. Adhikari, “Green Synthesis of Silver and Gold Nanoparticles Using Root Bark Extract of Mammea suriga: Characterization, Process Optimization, and Their Antibacterial Activity,” Bionanoscience, vol. 6, no. 2, pp. 110–120, Jun. 2016, doi: 10.1007/s12668-016-0199-8.
[53] P. Karuppaiya, E. Satheeshkumar, W.-T. Chao, L.-Y. Kao, E. C.-F. Chen, and H.-S. Tsay, “Anti-metastatic activity of biologically synthesized gold nanoparticles on human fibrosarcoma cell line HT-1080,” Colloids Surf B Biointerfaces, vol. 110, pp. 163–170, Oct. 2013, doi: 10.1016/j.colsurfb.2013.04.037.
[54] K. P. Kumar, W. Paul, and C. P. Sharma, “Green synthesis of gold nanoparticles with Zingiber officinale extract: Characterization and blood compatibility,” Process Biochemistry, vol. 46, no. 10, pp. 2007–2013, Oct. 2011, doi: 10.1016/j.procbio.2011.07.011.
[55] P. Singh, Y. J. Kim, C. Wang, R. Mathiyalagan, and D. C. Yang, “The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications,” Artif Cells Nanomed Biotechnol, pp. 1–8, Mar. 2015, doi: 10.3109/21691401.2015.1011809.
[56] K. P. Kumar, W. Paul, and C. P. Sharma, “Green synthesis of gold nanoparticles with Zingiber officinale extract: Characterization and blood compatibility,” Process Biochemistry, vol. 46, no. 10, pp. 2007–2013, Oct. 2011, doi: 10.1016/j.procbio.2011.07.011.
[57] M. Venkatachalam, K. Govindaraju, A. Mohamed Sadiq, S. Tamilselvan, V. Ganesh Kumar, and G. Singaravelu, “Functionalization of gold nanoparticles as antidiabetic nanomaterial,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 116, pp. 331–338, Dec. 2013, doi: 10.1016/j.saa.2013.07.038.
[58] M. Ateeq et al., “Green synthesis and molecular recognition ability of patuletin coated gold nanoparticles,” Biosens Bioelectron, vol. 63, pp. 499–505, Jan. 2015, doi: 10.1016/j.bios.2014.07.076.
[59] R. Manikandan et al., “Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 138, pp. 120–129, Mar. 2015, doi: 10.1016/j.saa.2014.10.043.
[60] S. Ghosh et al., “Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential,” J Nanobiotechnology, vol. 10, no. 1, p. 17, Dec. 2012, doi: 10.1186/1477-3155-10-17.
[61] M. R. Bindhu, P. Vijaya Rekha, T. Umamaheswari, and M. Umadevi, “Antibacterial activities of Hibiscus cannabinus stem-assisted silver and gold nanoparticles,” Mater Lett, vol. 131, pp. 194–197, Sep. 2014, doi: 10.1016/j.matlet.2014.05.172.
[62] D. S. Sheny, J. Mathew, and D. Philip, “Synthesis characterization and catalytic action of hexagonal gold nanoparticles using essential oils extracted from Anacardium occidentale,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 97, pp. 306–310, Nov. 2012, doi: 10.1016/j.saa.2012.06.009.
[63] N. Muniyappan and N. S. Nagarajan, “Green synthesis of gold nanoparticles using Curcuma pseudomontana essential oil, its biological activity and cytotoxicity against human ductal breast carcinoma cells T47D,” J Environ Chem Eng, vol. 2, no. 4, pp. 2037–2044, Dec. 2014, doi: 10.1016/j.jece.2014.03.004.
[64] C. Tamuly, M. Hazarika, and M. Bordoloi, “Biosynthesis of Au nanoparticles by Gymnocladus assamicus and its catalytic activity,” Mater Lett, vol. 108, pp. 276–279, Oct. 2013, doi: 10.1016/j.matlet.2013.07.020.
[65] N. Yang, L. WeiHong, and L. Hao, “Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and its in vitro cytotoxic effect on two normal cells,” Mater Lett, vol. 134, pp. 67–70, Nov. 2014, doi: 10.1016/j.matlet.2014.07.025.
[66] N. U. Islam, K. Jalil, M. Shahid, N. Muhammad, and A. Rauf, “Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities,” Arabian Journal of Chemistry, vol. 12, no. 8, pp. 2310–2319, Dec. 2019, doi: 10.1016/j.arabjc.2015.02.014.
[67] S. S. Godipurge et al., “A facile and green strategy for the synthesis of Au, Ag and Au–Ag alloy nanoparticles using aerial parts of R. hypocrateriformis extract and their biological evaluation,” Enzyme Microb Technol, vol. 95, pp. 174–184, Dec. 2016, doi: 10.1016/j.enzmictec.2016.08.006.
[68] C. Jayaseelan, R. Ramkumar, A. A. Rahuman, and P. Perumal, “Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity,” Ind Crops Prod, vol. 45, pp. 423–429, Feb. 2013, doi: 10.1016/j.indcrop.2012.12.019.
[69] K. Mohan Kumar, B. K. Mandal, M. Sinha, and V. Krishnakumar, “Terminalia chebula mediated green and rapid synthesis of gold nanoparticles,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 86, pp. 490–494, Feb. 2012, doi: 10.1016/j.saa.2011.11.001.
[70] S. Aswathy Aromal and D. Philip, “Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 97, pp. 1–5, Nov. 2012, doi: 10.1016/j.saa.2012.05.083.
[71] C. Jayaseelan, R. Ramkumar, A. A. Rahuman, and P. Perumal, “Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity,” Ind Crops Prod, vol. 45, pp. 423–429, Feb. 2013, doi: 10.1016/j.indcrop.2012.12.019.
[72] N. K. Kadiyala, B. K. Mandal, S. Ranjan, and N. Dasgupta, “Bioinspired gold nanoparticles decorated reduced graphene oxide nanocomposite using Syzygium cumini seed extract: Evaluation of its biological applications,” Materials Science and Engineering: C, vol. 93, pp. 191–205, Dec. 2018, doi: 10.1016/j.msec.2018.07.075.
[73] M. Guo, W. Li, F. Yang, and H. Liu, “Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 142, pp. 73–79, May 2015, doi: 10.1016/j.saa.2015.01.109.
[74] M. R. Bindhu, P. Vijaya Rekha, T. Umamaheswari, and M. Umadevi, “Antibacterial activities of Hibiscus cannabinus stem-assisted silver and gold nanoparticles,” Mater Lett, vol. 131, pp. 194–197, Sep. 2014, doi: 10.1016/j.matlet.2014.05.172.
[75] C. Tamuly, M. Hazarika, and M. Bordoloi, “Biosynthesis of Au nanoparticles by Gymnocladus assamicus and its catalytic activity,” Mater Lett, vol. 108, pp. 276–279, Oct. 2013, doi: 10.1016/j.matlet.2013.07.020.
[76] N. Yang, L. WeiHong, and L. Hao, “Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and its in vitro cytotoxic effect on two normal cells,” Mater Lett, vol. 134, pp. 67–70, Nov. 2014, doi: 10.1016/j.matlet.2014.07.025.
[77] R. D. Prasad et al., “Emerging Trends of Bioactive Nano-materials in Modern Veterinary Science and Animal Husbandry,” ES Food and Agroforestry, 2024, doi: 10.30919/esfaf1144.
[78] V. Thakur, A. Kumar, and J. Singh, “Mycobial Nanotechnology in Bioremediation of Wastewater,” in Microbes Based Approaches for the Management of Hazardous Contaminants, Wiley, 2024, pp. 1–11. doi: 10.1002/9781119851158.ch1.
[79] P. Manivasagan, J. Venkatesan, K.-H. Kang, K. Sivakumar, S.-J. Park, and S.-K. Kim, “Production of α-amylase for the biosynthesis of gold nanoparticles using Streptomyces sp. MBRC-82,” Int J Biol Macromol, vol. 72, pp. 71–78, Jan. 2015, doi: 10.1016/j.ijbiomac.2014.07.045.
[80] K. Kalishwaralal et al., “Biosynthesis of silver and gold nanoparticles using Brevibacterium casei,” Colloids Surf B Biointerfaces, vol. 77, no. 2, pp. 257–262, Jun. 2010, doi: 10.1016/j.colsurfb.2010.02.007.
[81] M. Girilal, A. Mohammed Fayaz, P. Mohan Balaji, and P. T. Kalaichelvan, “Augmentation of PCR efficiency using highly thermostable gold nanoparticles synthesized from a thermophilic bacterium, Geobacillus stearothermophilus,” Colloids Surf B Biointerfaces, vol. 106, pp. 165–169, Jun. 2013, doi: 10.1016/j.colsurfb.2012.12.038.
[82] P. Luo, Y. Liu, Y. Xia, H. Xu, and G. Xie, “Aptamer biosensor for sensitive detection of toxin A of Clostridium difficile using gold nanoparticles synthesized by Bacillus stearothermophilus,” Biosens Bioelectron, vol. 54, pp. 217–221, Apr. 2014, doi: 10.1016/j.bios.2013.11.013.
[83] A. K. Suresh et al., “Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis,” Acta Biomater, vol. 7, no. 5, pp. 2148–2152, May 2011, doi: 10.1016/j.actbio.2011.01.023.
[84] B. S. Srinath and V. Ravishankar Rai, “Rapid biosynthesis of gold nanoparticles by Staphylococcus epidermidis: Its characterisation and catalytic activity,” Mater Lett, vol. 146, pp. 23–25, May 2015, doi: 10.1016/j.matlet.2015.01.151.
[85] B. Nair and T. Pradeep, “Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains,” Cryst Growth Des, vol. 2, no. 4, pp. 293–298, Jul. 2002, doi: 10.1021/cg0255164.
[86] S. Baker and S. Satish, “Biosynthesis of gold nanoparticles by Pseudomonas veronii AS41G inhabiting Annona squamosa L.,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 150, pp. 691–695, Nov. 2015, doi: 10.1016/j.saa.2015.05.080.
[87] M. P. Patil et al., “Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines,” Colloids Surf B Biointerfaces, vol. 183, p. 110455, Nov. 2019, doi: 10.1016/j.colsurfb.2019.110455.
[88] V. K. Gupta et al., “Biosynthesis of silver nanoparticles using chitosan immobilized Bacillus cereus: Nanocatalytic studies,” J Mol Liq, vol. 188, pp. 81–88, Dec. 2013, doi: 10.1016/j.molliq.2013.09.021.
[89] A. Roychoudhury, S. Sarkar, and S. Chakraborty, “Fungal-mediated synthesis of gold and titanium nanoparticles and their application in agriculture,” in Myconanotechnology and Application of Nanoparticles in Biology, Elsevier, 2023, pp. 79–92. doi: 10.1016/B978-0-443-15262-7.00010-3.
[90] K. Seku, S. S. Hussaini, M. Radhakrishna Reddy, G. Bhagavanth Reddy, and K. Kishore Kumar, “Fungal-mediated synthesis of gold nanoparticles and their biological applications,” in Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications, Elsevier, 2023, pp. 23–58. doi: 10.1016/B978-0-323-99922-9.00011-8.
[91] A. Mishra, M. Kumari, S. Pandey, V. Chaudhry, K. C. Gupta, and C. S. Nautiyal, “Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp.,” Bioresour Technol, vol. 166, pp. 235–242, Aug. 2014, doi: 10.1016/j.biortech.2014.04.085.
[92] D. Philip, “Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 73, no. 2, pp. 374–381, Jul. 2009, doi: 10.1016/j.saa.2009.02.037.
[93] K. D. Lee, P. C. Nagajyothi, T. V. M. Sreekanth, and S. Park, “Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities,” Journal of Industrial and Engineering Chemistry, vol. 26, pp. 67–72, Jun. 2015, doi: 10.1016/j.jiec.2014.11.016.
[94] M. Owais et al., “Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer,” Int J Nanomedicine, p. 2305, Oct. 2011, doi: 10.2147/IJN.S23195.
[95] S. A. Kumar, Y.-A. Peter, and J. L. Nadeau, “Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin,” Nanotechnology, vol. 19, no. 49, p. 495101, Dec. 2008, doi: 10.1088/0957-4484/19/49/495101.
[96] S. S. Shankar, A. Ahmad, R. Pasricha, and M. Sastry, “Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes,” J Mater Chem, vol. 13, no. 7, p. 1822, 2003, doi: 10.1039/b303808b.
[97] E. Castro-Longoria, A. R. Vilchis-Nestor, and M. Avalos-Borja, “Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa,” Colloids Surf B Biointerfaces, vol. 83, no. 1, pp. 42–48, Mar. 2011, doi: 10.1016/j.colsurfb.2010.10.035.
[98] A. Mishra et al., “Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C2C12 cells,” Appl Microbiol Biotechnol, vol. 92, no. 3, pp. 617–630, Nov. 2011, doi: 10.1007/s00253-011-3556-0.
[99] R. Sanghi, P. Verma, and S. Puri, “Enzymatic Formation of Gold Nanoparticles Using &lt;i&gt;Phanerochaete Chrysosporium&lt;/i&gt;,” Advances in Chemical Engineering and Science, vol. 01, no. 03, pp. 154–162, 2011, doi: 10.4236/aces.2011.13023.
[100] K. B. Narayanan and N. Sakthivel, “Mycocrystallization of gold ions by the fungus Cylindrocladium floridanum,” World J Microbiol Biotechnol, vol. 29, no. 11, pp. 2207–2211, Nov. 2013, doi: 10.1007/s11274-013-1379-0.
[101] A. Karnwal et al., “Gold Nanoparticles in Nanobiotechnology: From Synthesis to Biosensing Applications,” ACS Omega, vol. 9, no. 28, pp. 29966–29982, Jul. 2024, doi: 10.1021/acsomega.3c10352.
[102] I. N. Rizki, W. Klaypradit, and Patmawati, “Utilization of marine organisms for the green synthesis of silver and gold nanoparticles and their applications: A review,” Sustain Chem Pharm, vol. 31, p. 100888, Apr. 2023, doi: 10.1016/j.scp.2022.100888.
[103] K. Deepa, A. Sridhar, and T. Panda, “Biogenic Gold Nanoparticles: Current Applications and Future Prospects,” J Clust Sci, vol. 34, no. 3, pp. 1163–1183, May 2023, doi: 10.1007/s10876-022-02304-8.
[104] A. K. Verma and P. Kumar, “On Recent Developments in Biosynthesis and Application of Au and Ag Nanoparticles from Biological Systems,” J Nanotechnol, vol. 2022, pp. 1–19, Jun. 2022, doi: 10.1155/2022/5560244.
[105] F. Namvar et al., “Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum,” Research on Chemical Intermediates, vol. 41, no. 8, pp. 5723–5730, Aug. 2015, doi: 10.1007/s11164-014-1696-4.
[106] B. Sharma et al., “Biosynthesis of gold nanoparticles using a freshwater green alga, Prasiola crispa,” Mater Lett, vol. 116, pp. 94–97, Feb. 2014, doi: 10.1016/j.matlet.2013.10.107.
[107] M. Ramakrishna, D. Rajesh Babu, R. M. Gengan, S. Chandra, and G. Nageswara Rao, “Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity,” J Nanostructure Chem, vol. 6, no. 1, pp. 1–13, Mar. 2016, doi: 10.1007/s40097-015-0173-y.
[108] F. Arockiya Aarthi Rajathi, C. Parthiban, V. Ganesh Kumar, and P. Anantharaman, “Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing),” Spectrochim Acta A Mol Biomol Spectrosc, vol. 99, pp. 166–173, Dec. 2012, doi: 10.1016/j.saa.2012.08.081.
[109] T. S. Dhas, V. G. Kumar, V. Karthick, K. Govindaraju, and T. Shankara Narayana, “Biosynthesis of gold nanoparticles using Sargassum swartzii and its cytotoxicity effect on HeLa cells,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 133, pp. 102–106, Dec. 2014, doi: 10.1016/j.saa.2014.05.042.
[110] N. Abdel-Raouf, N. M. Al-Enazi, and I. B. M. Ibraheem, “Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity,” Arabian Journal of Chemistry, vol. 10, pp. S3029–S3039, May 2017, doi: 10.1016/j.arabjc.2013.11.044.
[111] B. Sharma et al., “Biosynthesis of gold nanoparticles using a freshwater green alga, Prasiola crispa,” Mater Lett, vol. 116, pp. 94–97, Feb. 2014, doi: 10.1016/j.matlet.2013.10.107.
[112] M. Singh, R. Kalaivani, S. Manikandan, N. Sangeetha, and A. K. Kumaraguru, “Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga,” Appl Nanosci, vol. 3, no. 2, pp. 145–151, Apr. 2013, doi: 10.1007/s13204-012-0115-7.
[113] B. Sharma et al., “Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C.Ag. and antioxidant activity of biomatrix loaded nanoparticles,” Bioprocess Biosyst Eng, vol. 37, no. 12, pp. 2559–2565, Dec. 2014, doi: 10.1007/s00449-014-1233-2.
[114] P. RAJASULOCHANA, R. DHAMOTHARAN, P. MURUGAKOOTHAN, S. MURUGESAN, and P. KRISHNAMOORTHY, “BIOSYNTHESIS AND CHARACTERIZATION OF GOLD NANOPARTICLES USING THE ALGA Kappaphycus alvarezii,” Int J Nanosci, vol. 09, no. 05, pp. 511–516, Oct. 2010, doi: 10.1142/S0219581X10007149.
[115] R. D. Prasad et al., “A Review on Modern Characterization Techniques for Analysis of Nanomaterials and Biomaterials,” ES Energy & Environment, 2024, doi: 10.30919/esee1087.
[116] lbtihaj H. Ali, H. O. Saheb, L. S. Alhiti R, and A. A. Al- Fahham, “Spectroscopy: Types, Principles And Clinical Uses,” International Journal of Health & Medical Research, vol. 03, no. 07, Jul. 2024, doi: 10.58806/ijhmr.2024.v3i07n08.
[117] S. S. Razi, V. K. Nautiyal, and G. Hitkari, “UV–visible spectroscopy in biomedical nanotechnology,” in Analytical Techniques for Biomedical Nanotechnology, IOP Publishing, 2023, pp. 3-1-3–12. doi: 10.1088/978-0-7503-3379-5ch3.
[118] V. Dave, R. Sharma, C. Gupta, and S. Sur, “Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy,” Colloids Surf B Biointerfaces, vol. 194, p. 111151, Oct. 2020, doi: 10.1016/j.colsurfb.2020.111151.
[119] J. Simon, S. Udayan, E. S. Bindiya, S. G. Bhat, V. P. N. Nampoori, and M. Kailasnath, “Optical characterization and tunable antibacterial properties of gold nanoparticles with common proteins,” Anal Biochem, vol. 612, p. 113975, Jan. 2021, doi: 10.1016/j.ab.2020.113975.
[120] B. R. Khalkho et al., “Citrate functionalized gold nanoparticles assisted micro extraction of L-cysteine in milk and water samples using Fourier transform infrared spectroscopy,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 267, p. 120523, Feb. 2022, doi: 10.1016/j.saa.2021.120523.
[121] S. Sathiyaraj et al., “Biosynthesis, characterization, and antibacterial activity of gold nanoparticles,” J Infect Public Health, vol. 14, no. 12, pp. 1842–1847, Dec. 2021, doi: 10.1016/j.jiph.2021.10.007.
[122] S. Vijayaram et al., “Applications of Green Synthesized Metal Nanoparticles — a Review,” Biol Trace Elem Res, vol. 202, no. 1, pp. 360–386, Jan. 2024, doi: 10.1007/s12011-023-03645-9.
[123] L. S. Alhiti, R. A. Jawad, R. A. Abd Alwaahed, and H. M. Sobhi, “Study of the Effect of Thin Layer Thickness on the Structural Properties of Copper Phthalocyanine (CuPc) Films Prepared by Vacuum Thermal Evaporation Method,” Al-Kitab Journal for Pure Sciences, vol. 8, no. 01, pp. 81–91, Apr. 2024, doi: 10.32441/kjps.08.01.p8.
[124] A. Y. Yassin, “Synthesized polymeric nanocomposites with enhanced optical and electrical properties based on gold nanoparticles for optoelectronic applications,” Journal of Materials Science: Materials in Electronics, vol. 34, no. 1, p. 46, Jan. 2023, doi: 10.1007/s10854-022-09402-3.
[125] X. Gong et al., “An overview of green synthesized silver nanoparticles towards bioactive antibacterial, antimicrobial and antifungal applications,” Adv Colloid Interface Sci, vol. 323, p. 103053, Jan. 2024, doi: 10.1016/j.cis.2023.103053.
[126] G. Li, H. Zhang, and Y. Han, “Applications of Transmission Electron Microscopy in Phase Engineering of Nanomaterials,” Chem Rev, vol. 123, no. 17, pp. 10728–10749, Sep. 2023, doi: 10.1021/acs.chemrev.3c00364.
[127] Z. Lyu, L. Yao, W. Chen, F. C. Kalutantirige, and Q. Chen, “Electron Microscopy Studies of Soft Nanomaterials,” Chem Rev, vol. 123, no. 7, pp. 4051–4145, Apr. 2023, doi: 10.1021/acs.chemrev.2c00461.
[128] L. R. McCourt, B. S. Routley, M. G. Ruppert, and A. J. Fleming, “Feasibility of gold nanocones for collocated tip‐enhanced Raman spectroscopy and atomic force microscope imaging,” Journal of Raman Spectroscopy, vol. 55, no. 3, pp. 336–346, Mar. 2024, doi: 10.1002/jrs.6625.
[129] A. Karnwal et al., “Gold Nanoparticles in Nanobiotechnology: From Synthesis to Biosensing Applications,” ACS Omega, vol. 9, no. 28, pp. 29966–29982, Jul. 2024, doi: 10.1021/acsomega.3c10352.
[130] Z. Lyu, L. Yao, W. Chen, F. C. Kalutantirige, and Q. Chen, “Electron Microscopy Studies of Soft Nanomaterials,” Chem Rev, vol. 123, no. 7, pp. 4051–4145, Apr. 2023, doi: 10.1021/acs.chemrev.2c00461.
[131] A. M. Joshua, G. Cheng, and E. V. Lau, “Soft matter analysis via atomic force microscopy (AFM): A review,” Applied Surface Science Advances, vol. 17, p. 100448, Oct. 2023, doi: 10.1016/j.apsadv.2023.100448.
[132] Z. Lou, Y. Zhang, Y. Li, and L. Xu, “Study on microscopic physical and chemical properties of biomass materials by AFM,” Journal of Materials Research and Technology, vol. 24, pp. 10005–10026, May 2023, doi: 10.1016/j.jmrt.2023.05.176.
