*Adel A. M. Saeed1, Galal A. Ahmed2, and Ahmed T. A. Al-Sarahi1
1Chemistry Department, Faculty of Science, University of Aden, Yemen
Email: adel_saeed73@yahoo.com
2Chemistry Department, Faculty of Education, University of Aden, Yemen
Email: galalalbakshi@gmail.com
HNSJ, 2022, 3(2); https://doi.org/10.53796/hnsj3211
Published at 01/02/2022 Accepted at 17/01/2022
Abstract
Sulfur compounds represent one of the most common impurities present in crude oil. The combustion of these compounds in fossil fuels tends to release sulfur dioxide into the atmosphere, which leads to acid rain and reduces the life of the engine due to corrosion. The process of biodesulfurization rationally exploits the ability of certain microorganisms in the removal of sulfur prior to fuel burning, without loss of calorific value. The recent study focused on reducing the total sulfur of Al-Masila (Hadramout–Yemen) crude oil, by the biodesulfurization process using two sources of Pseudomonas aeruginosa bacterial strains (one isolated clinically from wounds and the other procured from American type culture collection, ATCC 27853). All the results showed that P. aeruginosa bacteria can be used to remove the sulfur from Yemeni crude oil.
Keywords: Biodesulfurization, Crude oil, P. aeruginosa
عنوان البحث
النزع الحيوي للكبريت في نفط خام المسيلة (حضرموت-اليمن) باستخدام بكتيريا الزائفة الزنجارية
عادل أحمد محمد سعيد1،*، جلال عبدالله أحمد عمر2، أحمد ثابت أحمد السرحي1
1 قسم الكيمياء، كلية العلوم، جامعة عدن، عدن، اليمن
Email: adel_saeed73@yahoo.com
2 قسم الكيمياء، كلية التربية، جامعة عدن، عدن، اليمن
Email: galalalbakshi@gmail.com
HNSJ, 2022, 3(2); https://doi.org/10.53796/hnsj3211
تاريخ النشر: 01/02/2022م تاريخ القبول: 17/01/2022م
المستخلص
تمثل مركبات الكبريت واحدة من مواد عدم نقاوة النفط الخام. يؤدي احتراق هذه المركبات إلى إطلاق ثاني أكسيد الكبريت إلى الغلاف الجوي، مما يؤدي إلى تكون المطر الحامضي وتقصير العمر الافتراضي للمحركات بسبب تآكلها. تستخدم عملية النزع الحيوي للكبريت باستخدام الكائنات المجهرية في إزالة الكبريت مع الحفاظ على القيمة السعرية قبل احتراق الوقود عملية بالغة الأهمية. ركزت الدراسة الحالية على اتباع الطريقة الحيوية لنزع الكبريت للتقليل من الكبريت الكلي في خام نفط المسيلة (حضرموت-اليمن) وذلك عبر استخدام مصدرين (معزولة سريريا من الجروح أو جاهزة) من بكتيريا الزائفة الزنجارية. اظهرت النتائج إمكانية استخدام هذه النوع من البكتيريا في نزع الكبريت من النفط الخام اليمني.
الكلمات المفتاحية: النزع الحيوي للكبريت، النفط الخام، الزائفة الزنجارية
1. INTRODUCTION
Petroleum can be defined as a complex mixture of hydrocarbons, non-hydrocarbons, and heteroatom-containing compounds (Overton et al., 2016). Petroleum consists of carbon, hydrogen, and heteroatoms like sulfur, nitrogen, oxygen, metals, etc. Among heteroatoms, sulfur is the most abundant with around 0.03–6 wt% in natural gas and crude oils (Bajia et al., 2017; Shahaby & Essam-El-din, 2017). When the total amount of sulfur is <0.42 %, it is called sweet crude oil, while when the amount of sulfur is more than around 0.42 %, it is called sour crude oil. Sulfur-containing compounds are classified into different types, as shown in Fig.1(Saleh, 2020). It is recommended that sulfur compounds are removed in the refining process as they cause the deactivation of the catalysts used in crude oil processing and corrosion problems in pipelines, along with the pumping, and refining equipment. From an environmental point of view, the sulfur left in fuels may cause the emission of toxic gases that react with water and cause acid rain. Thus, these gases or acid products can damage buildings and other materials (Das et al., 2020; Elwan et al., 2020; Jumina et al., 2021).
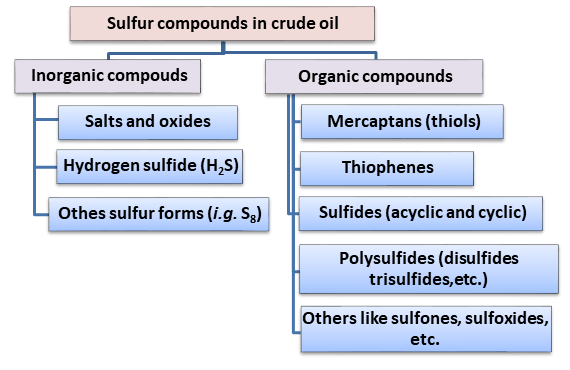
Fig. 1. Inorganic/organic sulfur compounds in crude oil (adopted from Saleh, 2020)
Currently, hydrodesulfurization (HDS) is employed to remove sulfur from fossil fuels. HDS operates at high temperature and pressure, and besides, it cannot remove completely sulfur (Nezammahalleh, 2015). Biodesulfurization (BDS) through microbial activities can solve this problem. Compared with the HDS process, the biodesulfurization (BDS) process using microorganisms and or enzymes could be carried out more safely, under mild conditions. This process of microbial desulphurization or biodesulfurization is expected to overcome the technical and economic problems associated with HDS as it has the potential benefits of lower capital and operating costs and will produce lesser greenhouse gases (Adlakha et al., 2016; Al-Bidry & Azeez, 2020).
Many microorganisms have been reported to use various petroleum hydrocarbons and sulfur compounds, as their sole carbon and energy substrate, despite their extreme insolubility in the aqueous phase. It is possible to desulfurize crude oil directly by selecting appropriate microbial species (Javadli & de Klerk, 2012). Numerous genera of bacteria are known as good hydrocarbon degraders such as Pseudomonas, Rhodococcus, Bacillus, Mycobacterium, Klebsiella, Enterobacter, Actinomycetes, Acinetobacter, etc. (Al-Zahrani & Idris, 2010; Ban et al., 2013; Bhatia & Sharma, 2012; Izumi et al., 1994; Kirimura et al., 2001; Raheb et al., 2010). However, biodesulfurization has become an alternative way to remedy crude oil and refined products, where the addition of specific microorganisms or enhancement of microorganisms already present, can improve desulfurizing efficiency (Austin & Callaghan, 2013). In order to develop environmental technologies for crude oil desulfurization, it is necessary to isolate and characterize specific microbial species for evaluation of their efficacy in the utilization of sulfur compounds before application to crude oil (Shahaby & Essam-El-din, 2017).
Sulfur removal through a biodesulfurization process could take place through C─C cleavage (Kodama pathway) or C─S cleavage (4S pathway) (Chen et al., 2019; Lateef et al., 2019). In the Kodama pathway, the biochemical reactions happen in the aromatic rings of dibenzothiophene as presented in Fig. 2 (a). Initially, the dibenzothiophene is oxidized to form cis-1- 2-dihydroxy-1,2-dihydro dibenzothiophene with the help of oxygen gas and NADH. The cis-1,2-dihydroxy-1,2-dihydro dibenzothiophene is then oxidized to form 1,2-dihydroxy dibenzothiophene. The 1,2-dihydroxy dibenzothiophene is oxidized again to form cis-4-[2-(3-hydroxy)- thionaphthenyl]-2-oxo-3-butenoic acid and afterward oxidized to pyruvic acid and 3-hydroxy-2-formylbenzothiophene. These two compounds are water-soluble and thus are spontaneously ejected from the crude oil phase (Das et al., 2020). In contrast, the sulfur element is removed as sulfite anion (SO3-2) from the aromatic hydrocarbon framework of dibenzothiophene in the 4S pathway Fig. 2 (b). At the start, dibenzothiophene-sulfoxide forms by oxidation dibenzothiophene compound and finally leads to produce dibenzothiophene sulfone in the presence of NADH, FMNH2, DszC, and DszD enzymes. Dibenzothiophene-sulfone compound is further oxidized to form 2-hydroxybiphenyl-2-sulfinate in the presence of oxygen gas, FMNH2, NADH, DszA, and DszD enzymes. Subsequently, the 2-hydroxybiphenyl-2-sulfinate is hydrolyzed to 2-hydroxybiphenyl and sulfite anion with the help of DszB enzymes. The 2-hydroxybiphenyl is a valuable by-product that can be used as a disinfectant and fungicide (Jumina et al., 2021).
Fig. 2. (a) Kodama and (b) 4S pathways on the biodesulfurization of dibenzothiophene (Jumina et al., 2021)
This work represents a continuation of our research in petroleum biodegradation technology. The study aims to use/isolate Pseudomonas aeruginosa bacteria. In addition, describing the ability of the selected bacterial strains to desulfurize crude oil and its refined products, and comparing the isolated local strains with reference commercial strains.
2. MATERIAL AND METHODS
2.1 Material
2.1.1 Microorganisms
The microorganisms used in this study were clinically isolated as characterized in Table (1).
Table (1). The categorizations of microorganisms
| Microorganisms | Isolated and diagnosed by |
| P. aeruginosa (from wounds) | Laboratories of The Supreme Board of Drug & Medical Appliances- Aden. |
| P. aeruginosa ATCC 27853 | Doctors Without International Borders -Yemen. |
2.1.2 Crude oil
Al-Masila crude oil, Hadramout –Yemen. Some chemical and physical properties are given in Table (2).
Table (2). Some properties of Al-Masila crude oil
| Test description | Test method | Result |
| Gravity | (D-4052 ASTM, 2019)1 | 33.1 |
| Specific Gravity at 15.5 °C | (D-4052 ASTM, 2019) | 0.8599 |
| Vapor Pressure (kPa) | (D-5191 ASTM, 2019) | 5.1 |
| Total Sulfur (wt %) | ( D-4294 ASTM, 2019) | 0.4015 |
| Carbon Residue (wt %) | (D-189 ASTM, 2019) | 0.25 |
| Pour Point 0C | (D-97 ASTM, 2019) | -11 |
| Kinematic Viscosity at 400C mm2/s | (D-445 ASTM, 2019) | 4.897 |
| Water Content (Vol %) | (D-95 ASTM, 2019) | 0.05 |
| Element Concentration (ppm)2
Vanadium (V) Nickel (Ni) Lead (Pb) Sodium (Na) Calcium (Ca) Zinc (Zn) Iron (Fe) Magnesium (Mg) Copper (Cu) |
IP 501(IP, 2019)3 | 25
13 NIL 3 1 NIL 2 1 NIL |
1ASTM: American Society for Testing and Materials; 2ppm: part per million; 3IP: Institute of Petroleum.
2.2 Method of Analysis
The total sulfur content of the untreated and treated crude oil samples was determined by SELFA-2800 sulfur-in-oil analyzer (Horiba, USA). The test method is based on ASTM D-4294 (ASTM, 2019). All the experiments of total sulfur measurements were performed at Aden Refinery Company’s laboratory. The concentrations of some elements (Table 2) in the studied crude oil were determined using inductively coupled plasma hyphenated to optical emission spectrometry (ICP-OES) model Thermo Scientific iCAP 6000 Series, USA at Central Processing Facility Laboratory of Al-Masila Petroleum Exploration and Production Company, Hadramout –Yemen.
2.2.1 Preparation of nutrient broth media (NB)
13.0 g of nutrient broth powder was added into one liter of distilled water in a flat-bottomed conical flask. The mixture was heated with frequent agitation and boiled for one minute to completely dissolve the media. The flask was then tightly closed using cotton wool and further covered with aluminum foil. The mixture was autoclaved for 15 minutes at 120±1˚C after which it was left to cool down to room temperature.
2.2.2 Experimental procedure
By using a sterile cotton swab, a few bacterial groups were taken and put into the test tube containing sterile saline water, and the turbidity was adjusted to meet 0.5 McFarland standard which contains approximately 1.5×108 colony formation unit/ mL(CFU) of bacteria (Tan, 2015). and was taken using a 1ml pipette to a 250 mL Erlenmeyer flask containing 50 mL of sterilized nutrient broth media. The contents were shaken to hydrolyze and then allowed to stay for 6 hours. For the growth, about 1 mL of broth was sampled after 6 h to measure the optical density of cell growth with a spectrophotometer at 600 nm (Hidayat et al., 2017). After that, 10 ml of crude oil was added and incubated at 30±1 °C. The sulfur content was measured at different times.
3. RESULTS AND DISCUSSION
3.1 Effects of Cell Concentration
To study the effect of Pseudomonas aeruginosa concentration on the desulfurization rate of Al-Masila crude oil with a starting concentration of 0.4015wt%, the reaction solution containing 50 mL of sterile nutrient broth medium, 10 mL of crude oil, and a known amount of two types of P. aeruginosa bacteria were tested for 24h. First type isolated from wounds in the laboratories of the Supreme Board of Drug & Medical Appliances- Aden. The second type obtained from Doctors Without International Borders -Yemen is (P. aeruginosa ATCC 27853). The reaction solution was centrifuged to separate the oil from the aqueous phase after the end of the reaction.
Figure 3 indicates the removal of sulfur from Al-Masila crude oil with different concentrations of bacteria. There is no significant difference between the two types of bacteria. The highest percentage of sulfur removal, about 48.5% at a concentration of 1.3 x 108 CFU, was coined by isolated bacteria of the wounds, while the highest percentage of removal sulfur by P. aeruginosa ATCC 27853 was 39.8 % at a concentration of 1.3 x 108 CFU.
Sulfur crude oil is often found buried in supra structures formed by several aromatic rings, which are linked by aliphatic bridges. Possibly, all the easily accessible sulfur was utilized in the round of desulfurization and the available sulfur in crude oil was either buried or difficult for uptake and desulfurization by the bacterium (Adlakha et al., 2016).
Fig. 3. Effects of P. aeruginosa cell concentrations on the desulfurization
3.2 Effect of Time
Figure 4 represents the effect of different periods on the desulfurization of Al-Masila crude oil using Pseudomonas aeruginosa. When 10 ml of the crude oil sample and 50 mL of sterile nutrient broth medium were treated with 1.5 x108 CFU P. aeruginosa (wounds) and P. aeruginosa ATCC 27853, the sulfur removal rate from the crude oil was 48.2 % and 41.2 %, respectively, and after 48h the removal rate was 53. 9% and 44.3%, correspondingly. Nevertheless, after 72h, there was no significant change in the results where the percentage of sulfur removal was 54% and 47%, respectively. It is noted that the percentage of the removed sulfur from crude oil was high by bacteria isolated from wounds, and the highest results were obtained after 24h. After that, the percentage of removal sulfur was low.
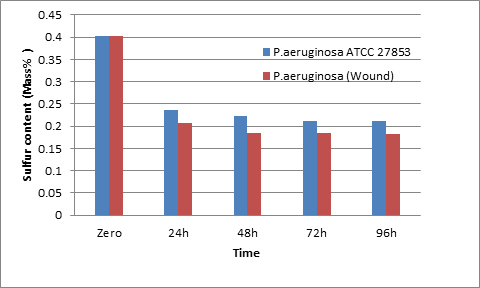
Fig .4. Final sulfur content measured at zero, 24, 48, 72, and 96 h desulfurization by P. aeruginosa
4. CONCLUSION
In contrary to the previous study (Saeed & Ahmed, 2021), this research applied P. aeruginosa bacteria to eradicate sulfur from Yemeni crude oil. The bacterial species decreases the total sulfur content of the crude oil to about 54% during 24h. Biodesulfurization offers an attractive alternative to conventional hydrodesulfurization due to the mild operating conditions and reaction specificity afforded by the biocatalyst. Nonetheless, further research in characterizing the kinetics of desulfurization and development of strain has to be carried out before realistic assessments in pilot plant studies.
REFERENCES
Adlakha, J., Singh, P., Ram, S. K., Kumar, M., Singh, M. P., Singh, D., Sahai, V., & Srivastava, P. (2016). Optimization of conditions for deep desulfurization of heavy crude oil and hydrodesulfurized diesel by Gordonia sp. IITR100. Fuel, 184, 761–769. https://doi.org/10.1016/j.fuel.2016.07.021
Al-Bidry, M. A., & Azeez, R. A. (2020). Removal sulfur components from heavy crude oil by natural clay. Ain Shams Engineering Journal, 11(4), 1265–1273. https://doi.org/10.1016/j.asej.2020.03.010
Al-Zahrani, A. A., & Idris, G. M. A. (2010). Biological treatment of hydrocarbon contaminants: petroleum hydrocarbon uptake by Pseudomonas alkanolytica. JKAU: Eng. Sci, 21(1), 39–53.
ASTM D-4052. (2019). Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. Annual Book of ASTM Standards.
ASTM, D-4052. (2019). Standard Test Method for Density and Relative Density of Liquids by Digital Density Meter. Annual Book of ASTM Standards, 05.02, 1–5.
ASTM D-4294. (2019). Standard Test Method for Sulfur in Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. Annual Book of ASTM Standards, i, 1–12. https://doi.org/10.1520/D2622-10
ASTM, D-445. (2019). Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (the Calculation of Dynamic Viscosity). Manual on Hydrocarbon Analysis, 6th Edition, 126-126–128. https://doi.org/10.1520/mnl10842m
ASTM, D-95. (2019). Standard Test Method for Water in Petroleum Products and Bituminous Materials by Distillation. Annual Book of Standards ASTM.
ASTM, D-97. (2019). Standard Test Method for Pour Point of Petroleum Products. Manual on Hydrocarbon Analysis, 6th Edition, 87-87–88. https://doi.org/10.1520/mnl10835m
ASTM, D.-5191. (2019). Standard Test Method for Vapor Pressure of Petroleum Products (Mini Method). Annual Book of ASTM Standards.
ASTM, D. (2019). Standard Test Method for Carbon Residue of Petroleum Products. Manual on Hydrocarbon Analysis, 6th Edition, 4(11), 144-144–148. https://doi.org/10.1520/mnl10846m
Austin, R. N., & Callaghan, A. V. (2013). Microbial enzymes that oxidize hydrocarbons. Frontiers in Microbiology, 4, 338.
Bajia, S. C., Singh, R. J., Bajia, B., & Kumar, S. (2017). Determination of sulfur content in petroleum products – an overview. 5993(March). https://doi.org/10.1080/17415993.2017.1289530
Ban, L.-L., Liu, P., Ma, C.-H., & Dai, B. (2013). Deep extractive desulfurization of diesel fuels by FeCl3/ionic liquids. Chinese Chemical Letters, 24(8), 755–758.
Bhatia, S., & Sharma, D. K. (2012). Thermophilic desulfurization of dibenzothiophene and different petroleum oils by Klebsiella sp. 13T. Environmental Science and Pollution Research, 19(8), 3491–349.
Das, P., Barbora, L., & Moholkar, V. S. (2020). Microbial desulphurization of refractory organic sulphur compounds from transportation fuels. Alternative Fuels and Their Utilization Strategies in Internal Combustion Engines, 311–329.
Elwan, H. A., Zaky, M. T., Farag, A. S., Soliman, F. S., & Hassan, M. E. D. (2020). Efficient pyridinium-based ionic liquid for deep oxidative desulfurization of model oil. Journal of Molecular Liquids, 113146.
Hidayat, M. Y., Saud, H. M., & Samsudin, A. A. (2017). Isolation and characterisation of sulphur oxidizing bacteria isolated from hot spring in Malaysia for biological deodorisation of hydrogen sulphide in chicken manure. Media Peternakan, 40(3), 178–187.
Izumi, Y., Ohshiro, T., Ogino, H., Hine, Y., & Shimao, M. (1994). Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Applied and Environmental Microbiology, 60(1), 223–226.
Javadli, R. & de Klerk, A. (2012). Desulfurization of heavy oil–oxidative desulfurization (ODS) as potential upgrading pathway for oil sands derived bitumen. Energy & Fuels, 26(1), 594–602.
Jumina, Kurniawan, Y. S., Purwono, B., Siswanta, D., Priastomo, Y., Winarno, A., & Waluyo, J (2021). Science and Technology Progress on the Desulfurization Process of Crude Oil.Bulletin of the Korean Chemical Society, 42(8), 1066–1081. https://doi.org/10.1002/bkcs.12342
Kirimura, K., Furuya, T., Nishii, Y., Ishii, Y., Kino, K., & Usami, S. (2001). Biodesulfurization of dibenzothiophene and its derivatives through the selective cleavage of carbon-sulfur bonds by a moderately thermophilic bacterim Bacillus subtilis WU-S2B. Journal of Bioscience and Bioengineering, 91(3), 262–26.
Nezammahalleh, H. (2015). Biodesulfurization of light crude oil using Bacillus subtilisWb600. Middle East, 1(3.0), 50–53.
Overton, E. B., Wade, T. L., Radović, J. R., Meyer, B. M., Miles, M. S., & Larter, S. R. (2016). Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography, 29(3), 50–63.
Raheb, J., Hajipour, M. J., & Memari, B. (2010). Increasing of biodesulfurization activity of newly recombinant Pseudomonas aeruginosa ATCC 9027 by cloning the flavin reductase gene. International Journal of Biotechnology & Biochemistry, 6(2), 219–230.
Saeed A.A.M. & Ahmed G.A. (2021). Total sulfur determination in AL-Masila (Hadramout–Yemen) crude oil after desulfurization using oxidative process. Research and Reviews: Journal of Environmental Sciences, 3(2), 1-9. https://doi.org/10.5281/zenodo.5553769
Saleh, T. A. (2020). Characterization, determination and elimination technologies for sulfur from petroleum: Toward cleaner fuel and a safe environment. Trends in Environmental Analytical Chemistry, 25. https://doi.org/10.1016/j.teac.2020.e00080
Shahaby, A. F., & Essam-El-din, K. M. (2017). Desulfurization of crude oil and oil products by local isolated bacterial strains. Int. J. Curr. Microbiol. Appl. Sci, 6, 2695–2711.
Tan, Y. K. (2015). Phytochemicals screening and antibacterial activity of Andrographics paniculata. UTAR. Google Scholar.
