Ahmed Thabet Ahmed Alsarahe1 , Afaf Mohammed Ali Saeed2
1Department of Chemistry, Faculty of Science, Aden University, Yemen.
Email: ahmedbiochem@gmail.com
2Department of Chemistry, Faculty of Science, Aden University, Yemen.
HNSJ, 2022, 3(2); https://doi.org/10.53796/hnsj3241
Published at 01/02/2022 Accepted at 25/01/2022
Abstract
Background: Vitamin C is one of the essential vitamins for humans and animals.
Objective: The aim of this study is to determine the concentration of vitamin C in some local and imported citrus fruits (oranges, strawberries). And local and imported non-citrus fruits (apples) that were randomly purchased from the markets of Aden Governorate (Yemen).
Method: Two types of citrus fruits and a non-citrus fruit were selected between local and imported fruits and analyzed by titration using (DCPIP) and HPLC method.
Results: The results of the current study indicated that the concentration of vitamin C in local oranges was (9.28 mg / 100 ml) and in imported oranges (9.18 mg / 100 ml), respectively, and in local strawberries (8.87 mg / 100 ml) and in imported strawberries (5.88 mg / 100 ml), while in imported and local apples (5.74 mg/100 ml) (4.41 mg/100 ml) respectively, Orange is the best source of vitamin C and Strawberry and Apple have lower content. Significant differences were observed in vitamin C of samples by both methods. titration and HPLC method are suitable for the determination of vitamin C, however, the HPLC method is more accurate, precise and specific.
Conclusion: We recommend eating citrus and non-citrus fruits to meet the daily requirement of vitamin C .
Key Words: Vitamin C, Titrimetric (DCPIP) , HPLC method, citrus and non-citrus fruits.
عنوان البحث
تحديد تركيز فيتامين ج في بعض الفواكه الحمضية وغير الحمضية بطريقتي المعايرة والكروماتوغرافيا السائلة عالية الأداء (HPLC)
احمد ثابت احمد السرحي1* عفاف محمد علي سعيد2
1 قسم الكيمياء – كلية العلوم – جامعة عدن – اليمن-1
بريد الكتروني: ahmedbiochem@gmail.com
قسم الكيمياء – كلية العلوم – جامعة عدن – اليمن.
HNSJ, 2022, 3(2); https://doi.org/10.53796/hnsj3241
تاريخ النشر: 01/02/2022م تاريخ القبول: 25/01/2022م
المستخلص
الخلفية: فيتامين ج هو احد الفيتامينات الاساسية للإنسان والحيوان
الهدف: الهدف من هذه الدراسة هو تحديد تركيز فيتامين ج في الحمضيات المحلية والمستوردة (البرتقال والفراولة). والفاكهة غير الحمضية المحلية والمستوردة (التفاح) التي تم شراؤها بشكل عشوائي من أسواق محافظة عدن (اليمن)
الطريقة: تم اختيار نوعين من الحمضيات وفاكهة غير حمضية بين الفاكهة المحلية والمستوردة وتحليلها بالمعايرة باستخدام (DCPIP)وطريقة HPLC
النتائج: أشارت نتائج الدراسة الحالية إلى أن تركيز فيتامين ج في البرتقال المحلي كان (9.28 جم / 100 مل) وفي البرتقال المستورد (9.18 جم / 100 مل) على التوالي وفي الفراولة المحلية (8.87 جم / 100 مل). ) وفي الفراولة المستوردة (5.88 جم / 100 مل). بينما في التفاح المستورد والتفاح المحلي (5.74 جم / 100 مل) (4.41 جم / 100 مل) على التوالي ، يعتبر البرتقال الأفضل مصدر لفيتامين سي والفراولة والتفاح يحتويان على نسبة أقل. فروق ذات دلالة إحصائية لوحظت في عينات فيتامين ج بكلتا الطريقتين. كلتا الطريقتين مناسبتان لـ تحديد فيتامين ج ، ومع ذلك ، فإن طريقة HPLC أكثر دقة ومحددة.
الخلاصة: نوصي بتناول الفواكه الحمضية وغير الحمضية لتلبية الاحتياجات اليومية من فيتامين ج.
الكلمات المفتاحية: فيتامين ج , المعايرة باستخدام (DCPIP) , طريقة HPLC , الفواكه الحمضية وغير الحمضية
INTRODUCTION:
Fruits and their juice are becoming an important part of the modern diet in many communities .They are nutritious and plays a significant role in a healthy diet because they offer good taste and a variety of nutrients found naturally in them. [1]
Vitamins are organic micronutrients mainly synthesized by plants and microorganisms, which do not provide energy. Animals are not able to synthesis them, consequently, these essential micronutrients must be supplied by the diet in small amounts or even trace amounts (micrograms or milligrams per day) for the maintenance of the metabolic functions of most animal. [2][3]
Vitamin C (chemical names: ascorbic acid and ascorbate) is a water-soluble vitamin,It is a six-carbon lactone which is synthesized from glucose by many animals and plants . Vitamin C is synthesized in the liver in some mammals and in the kidney in birds and reptiles, It is lactose-2,3,-deanol-L-gluconic acid which is odorless, a white solid with the chemical formula (C6H8O6) and the molecular weight is 176.13g/mole. [4]
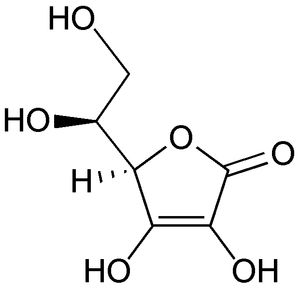
Figure 1. Structural formula for L-Ascorbic Acid
Vitamin C occurs naturally in food in two forms , The reduced form (Ascorbic acid) , and the oxidized form (Dehydroascorbic acid) ,[5][6][7][8][9][10] Both ascorbic acid and dehydroascorbic acid have vitamin activity [11][ 12].
As water – soluble antioxidant , vitamin C is in A unique position to ” scavenge” aqueous peroxyl radicals before these destructive substances have a chance to damage the lipids . It works along with vitamin E, a fat – soluble antioxidant , and the enzyme glutathione peroxidase to stop free radical chain reactions .
Vitamin C can enhance the body’s resistance to an assortment of diseases . Including infectious disorders and many types of cancer . It strengthens and protects the immune system by stimulating the activity of antibodies and immune system cells such as phagocytes and neutrophils .
Vitamin C protects the DNA of the cells from the damage caused by free radicals and mutagens . It also prevent harmful genetic alterations within cells and protects lymphocytes from mutations to the chromosomes . Vitamin C may be especially important in this day and age of widespread environmental pollution because it combats the effects of many such toxins , including ozone , carbon monoxide , hydrocarbons , pesticides and heavy metals.
Due to a range of benefits for vitamin C, a number of international authorities have increased their dietary recommendations for vitamin C from those previously recommended on the basis of preventing scurvy , Although these recommendations generally take into account variations in requirements based on age, sex, pregnancy and lactation, and sometimes smoking status .[ 13]
In 2020 a study was conducted evaluating the results of treatment with dexamethasone and vitamin C in infected with COVID-19, While controlled trial in critically ill patients with sepsis-induced acute respiratory distress syndrome (ARDS), it is observed that the administration of intravenous (IV) vitamin C 200 mg/kg per day for four days lowered 28-day mortality . [14] From a study conducted in patients hospitalized with COVID-19. The recovery rate in this study seems to be high due to treatment with a high dose of vitamin C and dexamethasone. [15]
MATERIALS AND METHODS
3.1 Study area:
Aden governorate is located along the southern coast of Republic of Yemen. It lies at (12O 47‘N) latitude and (44O 58‘E) longitude it is a semi island. [16] Aden is about 363 kilometers far from the capital Sana’a. It occupied about 750 km2 and divided into eight districts. The population of Aden governorate is 684,322 (CSO, 2009 in Arabic).

Figure 2. Map of Aden governorate adapted by www.europa.uk.com.
Chemicals, Reagents and Apparatus:
The chemicals used for the experimental purpose in the present study includes, 2,6-dichlorophenol indophenol (DCPIP)(Qualikems, India), Methanol(BDH, England), metaphosphoric acid (HPO3)(USA), Oxalic Acid(BDH, England),glacial acetic acid(Biotech LTD), sodium bicarbonate NaHCO3(BDH, England).high performance liquid chromatography (HPLC) Instrumnt model perkinelmer ,company model flexar It was also used for specification measurements As a confirmatory method.
Preparation of fruit samples for analysis by titration method:
After washing and drying the fruits with a cloth cut each fruit in half, The apples and strawberries were mixed in an electric juicer (per kilo 100 ml of distilled water) and filtered using a piece of cloth.
Preparation of fruit samples for analysis by HPLC:
The homogenous solid sample was measured around 10 to 30g and mixed it with 60 to 80 ml 6% of metaphosphoric acid (HPO3) for one minute, As for the juice of the samples, it was mixed with 30 ml of metaphosphoric acid and mixed well until homogeneous, then the volume was completed to 100 ml, The obtained extracted was filtered through filtration paper and washed it for few times by using vacuum pump filtration. Next, the filtrate quantitatively transferred into a 100 ml volumetric flask and 6% of metaphosphoric acid (HPO3) was added up to 100 ml volumetric mark. All the sample solutions was filtered again through 0.45 μm syringe filter. After that, the samples were run in the HPLC system.
Determination of vitamin C in the fruit samples by titration method:
The vitamin C (ascorbic acid ) of each sample was determined by the 2,6-dichlorophenol indophenol(DCPIP) as following :
An aliquot of 20 ml fruits juice sample was mixed with the equivalent quantity of 6 % oxalic acid solution in a 100 ml volumetric flask . The mixture was shaken until all components have become homogenous , then filtered through filter paper No.1. Next, the filtrate quantitatively transferred into a 100 ml volumetric flask and 6% of Oxalic acid was added up to 100 ml volumetric mark. 5 ml of this solution was titrated immediately with the dye (DCPIP) solution until the pink endpoint color appears , (persisting for 15 seconds), The amount of dye used in the titration was determined volumetrically and used in the calculation of the vitamin C content mg/ 100ml in the fruit samples. [17][18].
Calculation:
The vitamin C (ascorbic acid ) content was calculated from value of the titration process and the dye titer illustrated in the equation below :
Mg of vitamin C per 100 ml juice = Dye Titer × Titration value ×
dilution factor (for this study measurement).
Where:
Titration value = volume of dye consumed by sample at equivalent point.
Determination of vitamin C in the fruit samples by HPLC:
The separations were carried out on Carbon 18 (C18) column of (25 x 0.46) µm, Temperature 30 0C and The mobile phase used was a mixture of methanol – water (50.950, v/v) with a flow ratebof 1 ml/min . The injection volume was 20 µl and the wave length of UV detection at 254 nm.
First of all , Washing the column by mobile phase for 45 min and then standard solutions were run , After that washing the column by mobile phase for 45 min to remove any trace of standard solution , Finally samples were run and their retention time was compared with standard solution.
Statistical Analysis:
Statistical analysis of the data was performed using T-test and ANOVA were applied to find the significant difference between the data by using the statistical program SPSS (version 21).linear regressions for calibration curves were determined using Microsoft Excel 2010.
RESULTS AND DISCUSSION:
A three Imported and Local fruits were used in the present study. The results of Vitamin C concentration obtained for the three different types of fruits are tabulated in the Table (1and 2),and figures (6 and 7)
From the obtained results of the present study among citrus fruits, orange possessed high concentration of vitamin C. One of the study reported that the ascorbic acid concentration of freshly prepared Orange juice by titration method were 58.30 mg/100 mL Fatin and Azrina (2017). While that vitamin C concentration of orange juice 43.61 mg/100 ml used HPLC technique to analyze the vitamin C content of Orange juice .[19]
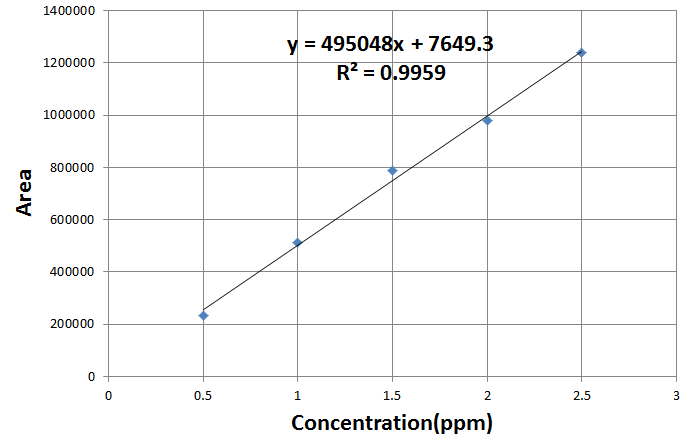
Preparation of the calibration curve of vitamin C:
Linearity: precision was determined by injecting 20μl Five standard spiked sample. (n = 5). The mean of the recorded peak area is taken for calibration curve The peak areas which were automatically measured by an integrator of HPLC instrument. The calibration curve obtained by plotting peak area against concentration of the standard and spiked samples in (Figure-2) which showed linearity in accordance to Beer’s law.[20][21]
Specificity:
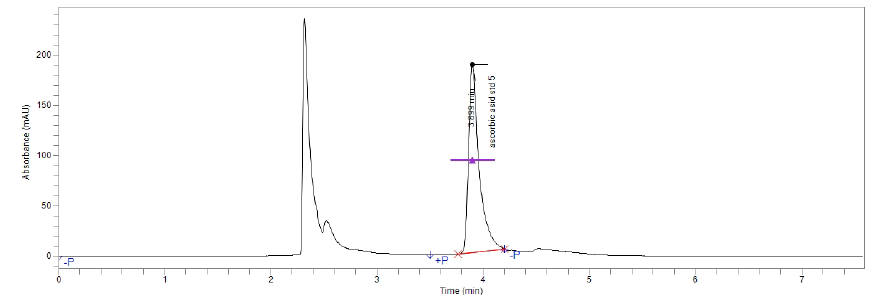
The specificity of the method was ascertained by analyzing standard ascorbic acid and sample. The retention time (RT) of ascorbic acid (vitamin C) confirmed by comparing the RT with that of the standard, which was within 4 minutes. The presence of other ingredients in the formulation did not cause any interface with Vitamin C peak so specific for analysis of Vitamin C.
Determination of(vitamin C)in the fruit samples by HPLC method :
Each of fruit samples solutions were treated for ascorbic acid determination. . These area values were referred to their related concentrations in the calibration curves (Table 1). The results HPLC reveal varying amounts of vitamin C in fruit samples. It was found that the imported oranges had the highest concentration compared to the rest of the fruits (9.2 mg / 100 ml), followed by the local orange (4.37 mg / 100 ml), followed by the local strawberry (2.15 mg / 100 ml), followed by the local apple (1.73 mg / 100 ml). Imported strawberries (1.51 mg / 100 ml) and the lowest concentration of vitamin C was in imported apples (1.37 mg / 100 ml), respectively. The results of the statistical analysis showed that the mean values of vitamin C differed significantly between citrus and non-acidic in favor of citrus at the level of statistical significance p <0.01.
Table(1): Concentration of vitamin C in fruit samples by HPLC method :
| Fruit samples | Peak Area | Vitamin C (mg/100ml) | Std. Deviation SD |
| Local Strawberry | 1071950.4 | 2.15 | 0.18 |
| Imported Strawberry | 759630.9 | 1.51 | 0.03 |
| Local apple | 864650.4 | 1.73 | 0.29 |
| Imported apple | 656395.5 | 1.37 | 0.28 |
| Local Orange | 2166883.2 | 4.37 | 0.93 |
| Imported Orange | 4537346.6 | 9.2 | 0.11 |
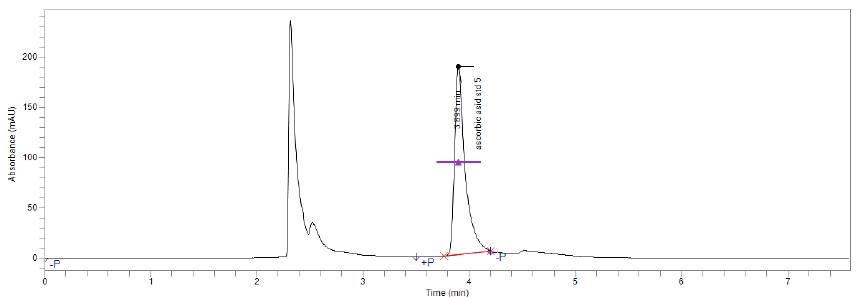
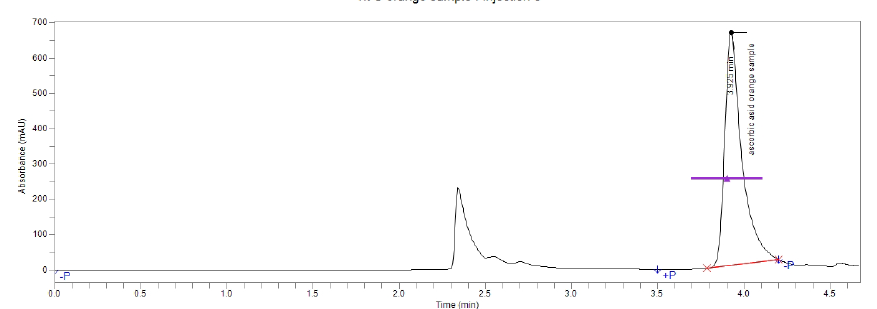
Figure(5):Chromatogram of vitamin C (Apple)
Figure(4):Chromatogram of vitamin C (Orange)
Figure(2):Calibration curve for vitamin Standard.
Figure(3):Chromatogram of vitamin C Standard
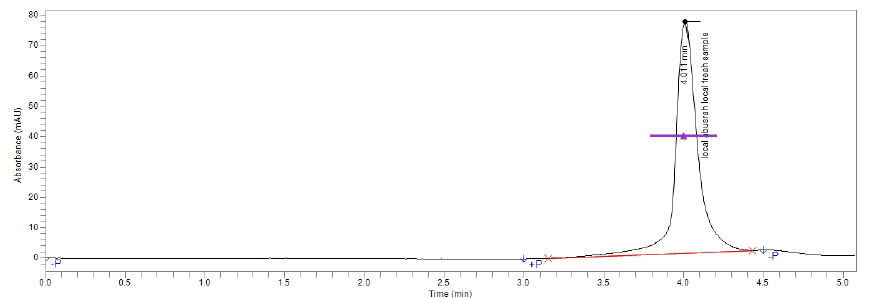
Figure(6):Chromatogram of vitamin C (strawberry)
Determination of (vitamin C)in the fruit samples by titration method :
Table 2 shows the total vitamin C concentration in fruit samples by titration method. By comparing the vitamin C the concentration of the three citrus fruit samples, can be seen that local orange remains to concentration the highest vitamin C of (9.54 mg/100ml) followedbyimportedoranges (9.18mg/100ml), localstrawberry(8.87mg/100ml), imported strawberry (5.88 mg/100ml),imported apple (5.74mg/100ml),and local apple(4.41 mg/100ml) Among the fruit samples, local apple has the lowest vitamin C content of only (4.41 mg/100ml). Similarly, the SD of the studied samples was within the acceptable range thus indicating the precision of the data. The concentrations of vitamin C in the fruit samples were also significantly different(p< 0.01).
Table(2): Concentration of vitamin C in fruit samples by titration method :
| fruit samples | Vitamin C(mg/100ml) | Std. Deviation SD |
| Imported Orange | 9.18 | 0.096 |
| Imported Strawberry | 5.88 | 0.05 |
| Imported Apple | 5.74 | 0.017 |
| Local Orange | 9.54 | 0.12 |
| Local Strawberry | 8.87 | 0.05 |
| Local Apple | 4.41 | 0.01 |
Differences in vitamin C concentration between titration and high-performance liquid chromatography (HPLC) methods:
Figures (6and7) shows a comparison concentration of vitamin C between titration and high performance liquid chromatography (HPLC) methods. There were significant differences in the content of vitamin C between both methods (p <0.01). Generally, the main advantage of using oxidation-reduction titration the method is because of its simplicity using simple equipment and inexpensive chemicals. Furthermore, the reaction of indophenol dye with the ascorbic acid is very fast. However, in some conditions, the oxidation-reduction titration may overestimate
he vitamin C content of fruit as the endpoint of titration could be difficult to be detected especially when high colored (e.g.,reddish-purplish)fruit was used Besides, the presence of reducing substances (ferrous ion, copper ion, sulphur dioxide, sulphite and thiosulphate) in the fruit samples can react with the indophenol dye and cause overestimation of vitamin C in fruit samples. When oxidation-reduction titration is not rapid, the exposure of samples to oxygen and light may cause degradation of the Table(1). Comparison of the vitamin C concentration in citrus fruit sample by titration method between present and previous studies ascorbic acids. Besides, the reduced ascorbic acids (dehydro ascorbic acids) also are not quantified in
this method. Therefore, the titration method is lack of specificity, do not overcome problem with reducing substances and might cause exposure to the air. High performance liquid chromatography (HPLC) method is a more specific, sensitive and selective technique for determining vitamin C concentration in fruit samples. Furthermore, HPLC method requires small amount of sample and chemicals, quite rapid and less susceptible to systemic error due to its high specificity.
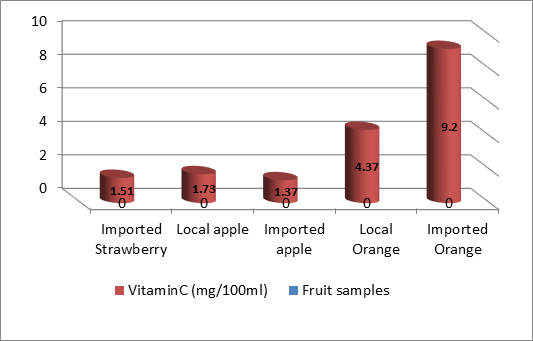
Figure(6):concentration of vitamin C By HPLC method .
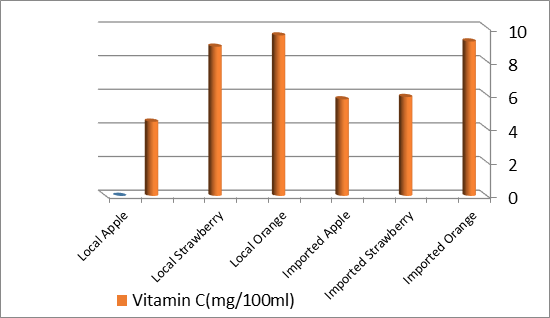
Figure(7):concentration of vitamin C By Titration method .
CONCLUSION:
In this study, orange contained the highest vitamin C among the citrus fruits followed by Strawberry, and apple by titration method. Similarly, orange also has the highest vitamin C by HPLC method followed by Strawberry and apple. Comparison between titration and HPLC method in terms of vitamin C content, showed significant differences in all fruit samples. The vitamin C contents in fruits samples were higher in titration method compared with HPLC method. The significant differences between the two methods could be affected due to many factors such as lack of specificity, presence of the reducing substances, time consuming and exposure to the air. Furthermore, the value of vitamin C content in HPLC method was lower than titration method. This could be due to the high sensitivity, selectivity and specificity of the HPLC method in isolating actual amount of vitamin C in fruit samples without any interference of other substances.
REFERENCES:
[1] Deanna, M.M. and J.S. Bland,( 2007). Acid – alkaline balance role in chronic disease and detoxification. Alternative Therapies, 13(4): 62-65
[2] Mora JR , Iwata My von Andrian UH,(2008) , “Vitamin effects on the immune system :Vitamin Aand D take centre stage “. Nature Reviews Immunology .8(9) : 685-698.
[3] Mataix .J, (2013), Vitamins I Nutricion para Educadores .Madrid .Diez de Santos .2nd ed : 117-126.
[4] Rickman, J.C, Bruhn, C.M, and Barrett DM. (2007).Nutritional Comparison of Fresh, Frozen, and Canned fruitsand Vegetables II. Vitamin A and Carotenoids, Vitamin E,Minerals and Fiber. Journal of the Science of Food and Agriculture; 87: 1185-1196.
[5] Diplock AT, Charleux JL, Crozier-Willi G, et al, (1998),” Functional food science and defence against reactive oxidative species”. Br J Nutr.:80(1): 77-112.
[6] Choi Y., Jeong H.S. and Lee J. (2007). Food Chem. (103):130-138.
[7] Kaviarasan S., Naik G H., Gangabagirathi R.,Anuradha C.V. and Priyadarshini K.I. (2007).Food Chem.(103):31-37.
[8] Seung K.Lee, Ader A.Kader , (2000). “Prehavest and Posthavest factors influencing vitamin C content of horticultural crops ” . Postharvest Biology and Technology 20 ,pp.207-220.
[9] Bendich A. (1987).Vitamin C and immune responses . Nutrition Tody , September , October , 21 (5) : pp. 26-30 .
[10]Bethlehem B. C. and Farrel T.(2013).Introduction to Organic and Biochemistry.8th ed.Brooks/Cole. CENGAGE Learning. Belmont. USA.
[11] Nagy, Steven . (1980). Vitamin C content of citurus fruit and their products : A review ,J. Agtic .Food Chem. 28(1),pp,8-18.
[12] Hernandez, Y., Lobo M. G, Gonzalez , (2006) .Determination of vitamin C in tropical fruits :Acomparative evolution of method . Food Chemistry 96,pp.654-664.
[13]Carr, A.C.; Lykkesfeldt, J.( 2020) Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr, 1–14. [CrossRef] [PubMed].
[14] Hemanth ,R.B ;Venkataramana ,K;Lakshmi,V.S,K and Tarun,.S (2020) Activities of Serum Ferritin and Treatment Outcomes Among COVID-19 Patients Treated With Vitamin C and Dexamethasone: An Uncontrolled Single-Center Observational Study. Journal Cureus. 12(11):PMC7732779.
[15] Horby, P. et al. (2020) Effect of dexamethasone in hospitalized patients withCOVID19:preliminaryreport.PreprintatmedRxivhttps://doi.org/10.1101/2020.06.22.20137273.
[16] Nasr.S.M ,Okbah .M.A,and Kasem.S.M(2006).Environmental Assessment of Heavy Metal Pollution in Bottom Sediments of Aden Port , Yemen.International Journal of Oceans and Oceanography, 1(1):99 – 109.
[17] AOAC. (1990). Method.Official Methods of Analysis of the Association of Official Analytical Chemistry,15th ,volume2.
[18] Masood.N (2007),The Effect of Storage on the Vitamin C Content of Some Citrus Fruits Cultivated in Modia -Abyan Governorate (Yemen),Faculty of Aden Education,Aden University,pp1-66.
[19] Fatin Najwa, and R. and Azrina, A. (2017) Comparison of vitamin C content in citrus fruits by titration and high performance liquid chromatography (HPLC) methods; International Food Research Journal 24(2): 726-733.
[20] Jenke, D.R., (1996). Hyphenated Techniques In Supercritical Fluid Chromatography And Extraction J. Liq. Chromat. Related Technol., 19: 737.
[21] Sabzevarizadeh, M. and H. Najafzadeh, (2012). Comparison Effect of Silymarin and Vitamin C on Liver Function in Myoglobinuric Status in Rats World Applied Sciences Journal, 17(2): 228-232.
