Najat O. A. Al-Salahia, Mohammed S. H. Al-Kahalib, Mohamed A. O. Al-Gubilic
a Chemistry Department, Faculty of Education, Abyan University, Yemen
b Chemistry Department, Faculty of Science, Sana’a University, Yemen
c Chemistry Department, Faculty of Education, Shabwah University, Yemen
HNSJ, 2023, 4(3); https://doi.org/10.53796/hnsj4336
Published at 01/03/2023 Accepted at 21/02/2023
Abstract
This research study uses the combination of the catalyst ZnO and sunlight to find the photo-degradation rate of Methomyl “ALFURAT” insecticide. The obtained results were very promising. It was found that the ZnO catalyst has speeded up the degradation very effectively in the presence of sunlight. The conditions of the experiment itself in the dark had no effect, nor did the sunlight have any effect without the presence of the catalyst in the decomposition of the pesticide, as the concentration of the pesticide remained almost constant. Because Methomyl insecticide, give maximum absorbance in the U.V. region at 234 nm wavelength, the absorbance measurements were taken at this wavelength. The absorbance decreased rapidly with time, an indication that the insecticide has underwent a rapid degradation process. The method was described within, the rate constant for the degradation process, and half-life were calculated. This simple method can have wider economical and environmental impacts. It can be applied in the treatment of wastewater.
Key Words: Methomyl, photocatalytic degradation, insecticide, sunlight, catalyst, zinc oxide, kinetics analysis, reaction mechanism.
1. Introduction
Methomyl (methyl N (methylcarbamoyloxy) ethanimidothioate) is Carbamates insecticide [1,2]. Its chemical structure is presented in (Figure 1) [3].
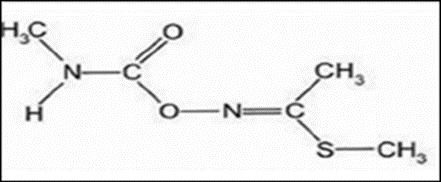
Fig 1: Chemical structure of Methomyl
Methomyl is highly soluble in water (57.9 g/L at 25 °C), has a low sorption affinity to soils and can therefore easily cause groundwater contamination in agricultural areas [4,5]. Methomyl, which has been classified by the World Health Organization, Environmental Protection Agency, USA, and European Commission as a very toxic and hazardous pesticide [5]. Methomyl is produced by reacting S-methyl-N-hydroxylthio acetamidate (MHTA) in methylene chloride with gaseous methyl isocyanate at 30–35 °C [4,6]. Methomyl is an oxime carbamate insecticide that controls a broad spectrum of arthropods such as spiders, ticks, moths, flies, beetles, aphids, leafhoppers, and spider mites often found on various field crops, ranging from fruits to tobacco [7]. Methomyl is used by farmers in Yemen to fight insects and pests in vegetables, fruits, cotton, citrus, tomatoes, and other crops.
Some work already has been done on degradation studies of Methomyl using ZnO photocatalysis [8,9], TiO2 photocatalysis [4,6] [8-13]. The aim of this study is to develop a cost-effective and eco-friendly technique by using a zinc oxide catalyst to treat a polluted aqueous environment with Methomyl pesticide.
2. Experimental
2.1 Apparatus
A Scinco is UV-Vis spectrophotometer, model: S -3100, Serial No S -3100-00-1303005U. Produced by SCINCO CO., LTD, equipped with a Software Version: LabProPlus 2.0 Bulid 5.
2.2 Materials
The commercial pesticides Methomyl (ALFURAT), (purity: 90%) was provided by Shanghai Agrofaith Industial Co.Ltd(China). The commercial pesticide Methomyl ―ALFURAT ‖ was supplied form the domestic market in Aden Governorate in the Republic of Yemen.
Zinc oxide (ZnO) (purity: 99%) was provided by Fizmerk India Chemcals.
2.3. Preparation of solutions
Methomyl (ALFURAT) stock solution (1×10-3 M) was prepared by preparing (1×10-2 M) from pesticide in distilled water then 10 ml was taken and diluting it to 100 ml. (the solution was kept in the dark.).
The catalyst solution was prepared by dissolving 0. 2g (ZnO) in (100 ml) de-ionized water [14].
2.4 Method
A concentration of 1 x 10-3 M of the commercial pesticide Methomyl was prepared initially. Then, the catalyst solution was prepared by dissolving 0.2g ZnO in (100 ml) de-ionized water. 2 ml of the 1 x 10-3 M insecticide solution was added into each of five 500 ml beakers. The beakers were taken to the roof, then the 0.2g/100ml ZnO catalyst solution were added into the five beakers to make up the total volume of 20 ml in each beaker, the mixtures were swirled, subjected to sunlight, and immediately the stopwatch was started. The first beaker (zero minute) was taken down for measurements, and the other mixtures were taken down for measurements every 10 minutes intervals. Since the insecticide gave a maximum absorbance at 234 nm for Methomyl, all the mixtures were measured at 234 nm for Methomyl. The catalyst solution was used as a blank. To avoid errors, the mixtures were filtered before measurements. The measurements were taken by a UV/Vis spectrophotometer. The average absorbance value of three readings was taken.
The same procedure was repeated twice, in the first case all the beakers containing the insecticide were subjected to sunlight without the adding the ZnO catalyst solution, but de-ionized water was added instead. In the second case all the beakers containing the insecticide/ZnO mixtures were placed in the dark [14].
3. Results and Discussions
3.1. Photocatalytic degradation
Absorbance of the insecticide/ZnO mixtures at 234 nm with time, the calculated concentrations of the insecticide with time, and the rate constants are shown in Table(1).
Table 1: Absorbance of insecticide /ZnO mixtures at 234 nm with time, the calculated concentrations of the insecticide with time, and the rate constants.
| Rate constant k
(min-1) |
Conc. of insecticide
(mol/L) |
Absorbance at
234 nm |
Time
(minutes) |
No. |
| – | 10-4×1.00 | 0.4826 | 0 | 1 |
| 0.0553 | 10-5×5.75 | 0.2777 | 10 | 2 |
| 0.0505 | 10-5×3.64 | 0.1755 | 20 | 3 |
| 0.0619 | 10-5×1.50 | 0.0751 | 30 | 4 |
| 0.0759 | 10-6×4.81 | 0.0232 | 40 | 5 |
Figure (2), shows the decrease in absorbance of the insecticide/ZnO mixtures at 234 nm with time which indicates that it underwent a photodegradation reaction.
Kinetic analysis of the photocatalytic process of Methomyl “ALFURAT” pesticide was determined, Absorbance of the insecticide/ZnO mixtures at 234 nm with time, the calculated concentrations of the insecticide with time, and the rate constants are shown in Tables (1 and 2). Beer-Lambert law was used to calculate the concentrations of the insecticide remaining at different times.
Table 2: Absorbance of “ALFURAT” /ZnO mixtures at 234 nm with time, the calculate half-life of the insecticide with time, ln c/c0 and percentage of Degradation.
| Degradation % | ln c/c0 | half-life t1/2 (min) | Absorbance at 234 nm | Time
(minutes) |
No. |
| – | 0 | – | 0.4826 | 0 | 1 |
| 43 | 0.55- | 12.5 | 0.2777 | 10 | 2 |
| 64 | -1.01 | 13.72 | 0.1755 | 20 | 3 |
| 85 | 1.86- | 11.20 | 0.0751 | 30 | 4 |
| 95 | -3.03 | 9.13 | 0.0232 | 40 | 5 |
Fig 2: The absorbance of “ALFURAT” /ZnO mixture with time in minutes at 234 nm, showing a decrease in absorbance with time.
A plot of ln (C/C0) versus irradiation time (t) is shown in (figure 3) for Methomyl “ALFURAT” pesticide degradation. It is quite clear that there exists a linear relation between ln (C/C0) and time. The pseudo first order rate constant for photo-degradation process k and R, linear regression coefficient with various con Methomyl “ALFURAT” pesticide centration is given in Table (1). From Tables (1and 2), the least square fit, R2 = 0.9626 and rate constant of degradation (K= 0.0609 min-1) was evaluated as evaluated. The linear relationship between the functions with large R2 values was observed [15].
Fig. 3: A plot of ln (C/C0) versus irradiation time of “ALFURAT” the first order model on photocatalysis of with catalyst (ZnO) under sun light, t = (0–40 min)
From Table (1) the percentage of the photocatalytic degradation of Methomyl “ALFURAT” pesticide with catalyst (ZnO) under sun light at 234 nm has been calculated during the different times (0 – 40 min) per ten minutes using following equation:
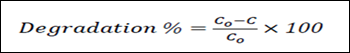 (1)
(1)
where Co is the initial pollutant concentration and C is the concentration of pollutant at time t [16,17].
The results showed that the percentage of the photocatalytic degradation of Methomyl “ALFURAT” pesticide was (95%) at (40) minute. the degradation percentage was higher at lower contaminant concentration.
Finally, the results of photocatalytic degradation of pesticide (Methomyl “ALFURAT”) under the influence of direct sunlight with catalyst(ZnO), this method has been carried out during different times of (10- 40) minutes The photocatalytic degradation method was very effective in the removal of pesticide (Methomyl “ALFURAT”).
Table 3: Comparing results of photocatalytic degradation of pesticide (Methomyl ―ALFURAT) /ZnO mixture with time
| pesticide | half-life t1/2
(min-1) |
Degradation % | Rate constant
k (min-1) |
SD | R2 |
| Methomyl
“ALFURAT” |
11.64 | 95 | 0.0609 | 0.009559 | 0.9626 |
SD=Standard deviation, R2 =Square of the correlation coefficient
From table (3) The obtained results indicate that the photocatalytic degradation of pesticide (Methomyl “ALFURAT”) is a first order reaction. The calculated values of the rate constant of degradation (K) were 0.0609 min-1 for Methomyl “ALFURAT”. with half-life of 11.64 minutes, this means This pesticide will disappear within one hour in presence of catalyst (ZnO) and under the influence of direct sunlight.
For the settings when the mixtures contained only insecticide without the ZnO catalyst solution, under sunlight, and the settings when the insecticide/ ZnO mixtures were placed in the dark, the absorbance measurements were not consistent and varied slightly up and down, but this did not represent a change in absorbance.
3.2. Possible Degradation Mechanism
The photocatalytic degradation of the pesticides take place on the surface of ZnO where •OH and O2•– radicals are trapped in the holes of reactive species. Oxygen and water are essential for photocatalytic degradation. The •OH radicals are strong enough to break the bonds in the pesticide molecules adsorbed on the surface of ZnO. The amount of ZnO and concentration of pesticides are constant, the number of •OH and O2•– radicals increases with the increase in the irradiation period and hence the pesticide molecules are completely degraded into smaller fragments [18-20].
The main possible reactions are presented in equations (2-5) [21,22] and equation (6):
ZnO + hv ⭢ ZnO + hvb+ + e–cb ( 2 )
OH– + hvb+ ⭢ •OH ( 3 )
H2O + hvb+ ⭢ •OH + H+ ( 4 )
O2 + e–cb ⭢ O2– ( 5 )
In the water purification by heterogeneous photocatalysis, the pollutants are usually organic. Their reaction in the presence of oxygen is: (equation 5) [23].
•OH + pollutants + O2⭢ CO2 + H2O + intermediate products ( 6 )
Oxygen is essential for complete degradation and should not be in competition at the level of adsorption with other reactive species on the catalyst.
there is an acceleration of the decrease in the concentration of the pollutant. Other research has shown that the rates and efficiencies of photo-assisted degradation of organic substrates are reported as significantly improved in the presence of oxygen or by the addition of several oxidizing species such as peroxydisulfate or peroxides [24-27]. This lacceleration may be related to inhibition of the recombination of electron-hole pair and also by the production of more radicals OH• in the middle, so we conclude that oxygen (O2) here plays the role of a catalyst but since it is not regenerated at the end of the reaction, it affects the performance of the degradation reaction by increasing, so we can conclude that reacts with the pollutant [24].
3.3. Proposed degradation pathway
In general, Methomyl degradation reaction can be expressed by the following equation which takes into account the absence of significant pH variation during the degradation.
C5H10O2N2S + 13/2 O2 →2NH4+ + SO42− + 5CO2 + H2O (7)
It is noteworthy that in general it is considered that ammonia is oxidized to nitrate after long irradiation time and reactions in which molecules are in their most oxidized state have been proposed [6,7].
Scheme 1: Tentative pathway for photocatalytic degradation of Methomyl
After the identification of the various by-products, a pathway of the photocatalytic degradation of Methomyl in water was tentatively proposed (Scheme 1) [6,25].
4. Conclusion
In this work, process was carried out under the influence of direct sunlight with catalyst (ZnO). This process has shown high efficiency in removal this pesticide (Methomyl “ALFURAT”). from this we conclude that the photocatalytic degradation process can not occur and be effective only in existing two factors together (light, catalyst).
The obtained results indicate that the photocatalytic degradation of pesticide is a first order reaction. The calculated values of the rate constant of degradation (K)was 0.0609 min-1 for pesticide. with half-life of 11.64 minutes, this means this pesticide will disappear within one hour in presence of catalyst (ZnO) and under the influence of direct sunlight.
Finally, the results of this study showed that the photocatalytic degradation process has high efficiency and effective in removal the pesticides from the aquatic environment, which could be applied to removing many organic pollutants in the sewage, and wastewater systems. This method is a simple, low-cost, safe and easy-to-handle.
References
- El-Saeid, M. H., BaQais, A., & Alshabanat, M. (2022). Study of the photocatalytic degradation of highly abundant pesticides in agricultural soils. Molecules, 27)634( 1-13.
- Lewis, K. A., Tzilivakis, J., Warner, D. J., & Green, A. (2016). An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment: An International Journal, 22(4), 1050-1064.
- EL-Saeid, M. H., Alotaibi, M. O., Alshabanat, M., AL-Anazy, M. M., Alharbi, K. R., & Altowyan, A. S. (2021). Impact of photolysis and TiO2 on pesticides degradation in wastewater. Water, 13(655(1-14.
- Tamimi, M., Qourzal, S., Assabbane, A., Chovelon, J. M., Ferronato, C., & Ait-Ichou, Y. (2006). Photocatalytic degradation of pesticide Methomyl: determination of the reaction pathway and identification of intermediate products. Photochemical & Photobiological Sciences, 5, 477-482.
- Strathmann, T. J., & Stone, A. T. (2001). Reduction of the carbamate pesticides oxamyl and Methomyl by dissolved FeII and CuI. Environmental science & technology, 35(12), 2461-2469.
- Malato, S., Blanco, J., Cáceres, J., Fernández-Alba, A. R., Agüera, A., & Rodrıguez, A. (2002). Photocatalytic treatment of water-soluble pesticides by photo-Fenton and TiO2 using solar energy. Catalysis Today, 76(2-4), 209-220.
- Kidd, H.& James, D. R. (1991). The agrochemicals handbook, Third Edition. Royal Society of Chemistry Information Services, Cambridge, England.
- Tomašević, A., Mijin, D., & Kiss, E. (2010). Photochemical behavior of the insecticide Methomyl under different conditions. Separation Science and Technology, 45(11), 1617-1627.
- Tomašević, A., Mijin, D., Gašic, S., & Kiss, E. (2014). The influence of polychromatic light on Methomyl degradation in TiO2 and ZnO aqueous suspension. Desalination and Water Treatment, 52(22-24), 4342-4349.
- Malato, S., Blanco, J., Vidal, A., Alarcón, D., Maldonado, M. I., Cáceres, J., & Gernjak, W. (2003). Applied studies in solar photocatalytic detoxification: an overview. Solar Energy, 75(4), 329-336.
- Oller, I., Gernjak, W., Maldonado, M. I., Pérez-Estrada, L. A., Sánchez-Pérez, J. A., & Malato, S. (2006). Solar photocatalytic degradation of some hazardous water-soluble pesticides at pilot-plant scale. Journal of Hazardous Materials, 138(3), 507-517.
- Juang, R. S., & Chen, C. H. (2014). Comparative study on photocatalytic degradation of Methomyl and parathion over UV-irradiated TiO2 particles in aqueous solutions. Journal of the Taiwan Institute of Chemical Engineers, 45(3), 989-995.
- Barakat, N. A. M., Nassar, M. M., Farrag, T. E., & Mahmoud, M. S. (2014). Effective photodegradation of Methomyl pesticide in concentrated solutions by novel enhancement of the photocatalytic activity of TiO 2 using CdSO 4 nanoparticles. Environmental Science and Pollution Research, 21, 1425-1435.
- Al-Shuja’aa, O. M., Al-Kahalib, M. S., & Al Dafa’e, B. (2013). Photodegradation of the Cypermethrin Form Insecticide “START” Induced by TiO2 in Aquatic Environment. Sana’a University Journal of Science & Technology, 5 (1), A(37-42).
- Bagwan, S. S. (2015). Photocatalytic Degradation of Hazardous Safranin (O) Dye by Using Self Synthesized TIO2 Nanoparticles. Master Thesis, National Institute of Technology Rourkela.
- Abduljabbar, A. A. M. (2013). Aquatic Photocatalytic and Thermal Degradation of some of Textile Dyes and Pesticides. Thesis Submitted for the Degree of Master in Chemistry (Physical Chemistry), Sana’a University, Republic of Yemen.
- Şayan, E. (2006). Optimization and modeling of decolorization and COD reduction of reactive dye solutions by ultrasound-assisted adsorption. Chemical Engineering Journal, 119(2-3), 175-181.
- Dehghani, M. H., & Fadaei, A. M. (2012). Photocatalytic oxidation of organophosphorus pesticides using zinc oxide. Research Journal of Chemistry and Environment, 16(3), 104-109.
- Pare, B., Jonnalagadda, S. B., Tomar, H., Singh, P., & Bhagwat, V. W. (2008). ZnO assisted photocatalytic degradation of acridine orange in aqueous solution using visible irradiation. Desalination, 232(1-3), 80-90.
- Fenoll, J., Ruiz, E., Hellín, P., Flores, P., & Navarro, S. (2011). Heterogeneous photocatalytic oxidation of cyprodinil and fludioxonil in leaching water under solar irradiation. Chemosphere, 85(8), 1262-1268.
- Yari, K., Seidmohammadi, A., Khazaei, M., Bhatnagar, A., & Leili, M. (2019). A comparative study for the removal of imidacloprid insecticide from water by chemical-less UVC, UVC/TiO 2 and UVC/ZnO processes. Journal of Environmental Health Science and Engineering, 17, 337-351.
- Zheng, W., Liu, W. P., Wen, Y. Z., & Lee, S. J. (2004). Photochemistry of insecticide imidacloprid: direct and sensitized photolysis in aqueous medium. Journal of Environmental Sciences, 16(4), 539-542.
- Konstantinou, I. K., & Albanis, T. A. (2003). Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways. Applied Catalysis B: Environmental, 42(4), 319-335.
- El Mouraille, N., Belmouden, M. & Ait Ichou, Y. (2018). UV/TiO2 photocatalytic oxidation of commercial pesticide in aqueous solution. American Journal of Innovative Research and Applied Sciences, 5(4) 36-43.
- Marinas, A., Guillard, C., Marinas, J. M., Fernández-Alba, A., Aguëra, A., & Herrmann, J. M. (2001). Photocatalytic degradation of pesticide–acaricide formetanate in aqueous suspension of TiO2. Applied Catalysis B: Environmental, 34(3), 241-252.
- Matthews, R. W. (1986). Photo-oxidation of organic material in aqueous suspensions of titanium dioxide. Water Research, 20(5), 569-578.
- Shifu, C., & Gengyu, C. (2005). Photocatalytic degradation of organophosphorus pesticides using floating photocatalyst TiO2· SiO2/beads by sunlight. Solar Energy, 79(1), 1-9.
