The Use of Microwave technology to prepare some organic compounds (Review Study)
Mariam Abdul-bary1, Jenan Al Ameri2
Department of Food Science, College of Agriculture, University of Basrah, Basrah, Iraq
Email: mariam.ouraiby@uobasrah.edu.iq
2Department of Chemistry, College of Science, University of Basrah, Basrah, Iraq
Email: jenan.mohamed@uobasrah.edu.iq
HNSJ, 2024, 5(11); https://doi.org/10.53796/hnsj511/25
Published at 01/11/2024 Accepted at 05/10/2024
Citation Methods
Abstract
Microwave technology has been introduced as a new technology applied within the scientific research arena, especially in the preparation of organic compounds. A high and pure percentage of product can be obtained via the use of such technology as well as such technology shortens the time of reaction and preparation compared with the old method used formerly in organic preparation the microwave-assisted synthesis process has revolutionized the world of chemical synthesis. This paper was prepared to discuss the various applications used to synthesize microwave-assisted organic compounds with more accuracy and focus on speed and scalability sides. Such a view becomes quite clear and obvious through this introduction regardless of the types of organic materials. Microwave-assisted synthesis provides a clean synthesis associated with enhanced reaction rates, higher selectivity and more economy to synthesize a large number of organic molecules, especially upon the application of microwave-assisted chemistry in place of the classical heating method. This paper introduces a brief of the reactions conducted with the use of microwave radiation. Such operations led to the use of catalyst–free or less catalyst reactions and smoothly recyclable solvents which are quite higher than the traditional method. By using such a method, we could economize the use of materials and equipment.
Key Words: Microwave, Organic Organs, Reaction Time.
Introduction:
A microwave oven is an electric device used in the heating of various types of food and such type of oven is unlike other common ovens since such microwave oven generates radiations called microwave used to heat food and it is called minor wave, infinitesimal wave or microwave. Minor waves or Microwaves are radiation or electromagnetic waves with a relatively short wavelength. Microwave technology was invented shortly before the Second World War. Microwaves have been used in the food industry since 1970. In the ’80s of the last century, research covering lab and industrial fields emerged and the first reaction of organic synthesis was published in 1986 (1). The tools used to manage such purposes had certain safety standards in order to use magnetic radiation. Microwave technology was basically used in chemical labs to synthesize and isolate natural materials and this method has substituted the classical extraction method using Soxhlet Extractor which requires quite a long time and huge quantities of solvents. One of the virtues of this method is the high temperature of solvent used in the extraction process because of pressure (2) that microwave radiation is deemed as one of the most significant practical and effective methods used in organic synthesis where the activity of many chemical reactions gets increased. Such technology has recently attracted the attention of researchers. In 1999, more than (40) researches were published and they covered many subjects including the effects of (3)microwave radiation on various aspects of Chemistry such as the reaction at superheating, interior non-thermal reactions and recombination reactions via free radicals. Moreover, the use of microwave radiation in the field of organic chemistry has presently occupied an increased attention which is considered one of the new methods used to increase the effect of chemical reactions. (4)(5)(6)(7)
Microwave Operation(8)
This device is operated by exposing food to a magnetic microwave where such microwaves penetrate foods and make water molecules and the molecules of other materials that exist in such foods to move after such molecules are exposed to microwaves, they start moving, vibrating, fractioning and crashing each other. Such status allows for such molecules to have temperature and energy and they get heated.(9)
The source of radiation of the microwave oven is the Maghetron tube which transfers electrical current to electromagnetic waves whose frequency is 2450 MHZ in most ovens. The frequency of the electrical current transferred through the said tube is 50 or 60 Hz. As to chemical reactions, most of the pioneering experiences of chemical composition were conducted with the use of microwaves conducted in local microwave ovens.(10) Yet, the developments of microwave equipment technology helped researchers to use a device used particularly for organic reactions.(11)
This article focuses on the major advances made in organic synthesis by using Microwave-assisted reactions with reactants clean and under solvent-free conditions. It also highlights the general characteristics of microwave applications in organic synthesis and shows that reactions under microwaves are fast, with an increase in production quantity and better selectivity.(12)
There are two types of such devices, they are: (13)
1 – Single-Mode Microwave. (14)
The Single-Mode Microwave is recognized by its capability of creating a constant wave which is brought up via an interference in spheres. Such interference generates a group of nodes where the microwave energy density is zero and another group whose microwave energy density is at its maximum levels. The factor that controls the design of such device is the distance from the sample to the magnet and such distance should be sufficient enough to place the sample properly at the maximum energy levels of the constant electromagnetic wave model.
2-Multi – Mode Microwave.
One of the basic features of this device is the intentional avoidance to generate an in–run constant wave model. The goal behind this is to generate the maximum level of jamming and chaos in the device. The more chaos is generated and radiation dispersion is magnified, the zone space due to the increase in the active warming in the device shall be likewise increased. (8)
1 –types organic reactions:
assisted organic reactions are widely classified into two classes: organic reactions using solvents and reactions under solvent–free conditions.
A – Organic Reactions Using Solvents
In the case of M.W–assisted reactions using (organic) solvents, reacting materials usually dissolve in the solvent which often actively associates with microwave ovens and thus it acts as an energy transmission medium. The use of water medium for reaction No. ( 15), where a minimum of 100 C temperature is used for compounds (16) aiming to make use of the hydrophobic characteristic that water relative static permittivity is 78 at 25o temperature and it gets down to 20 at 300o (17). Such value is converging for the purpose of comparison with the value of solvents use Acetone and then water can act as a safe possible environmental substitute of organic solvents as well as other environmental benefits that can be obtained out of the use of water instead of organic solvents. Below are some examples of reactions conducted with the use of solvents (18).
1 – Hydrolysis (19)
If C6H5CH2CL is dissolved in water in a microwave oven, water dissolution generates 97% of Benzyl Alcohol within 3 minutes while the regular water dissolution made by using the common method requires 35 minutes.

Fig. (1) Shows the hydrolysis of benzyl chloride using microwave radiation to form benzyl alcohol.
Benzamide regular water dissolution requires an hour but under microwave conditions, such water dissolution requires no more than 7 minutes to complete and generates 99% of Benzoic acid (20)
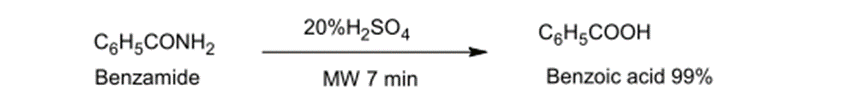
Fig. (2) Shows the hydrolysis of benzamide by microwave radiation to form benzoic acid.
2 – N – Acylations.
N-Acylations were conducted with the use of secondary amines and Isocyanates in CH2Cl2 under microwave radiation (8-10) minutes and a production yield of 94%. (4)
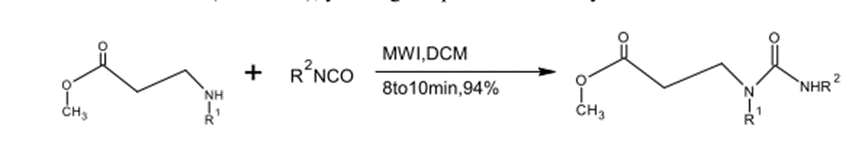
Fig. (3) Shows the reaction of the amine with the isocyanate under microwave irradiation.
3 – Cycloaddition (21)
Diplor Cycloaddition – 1,3 was prepared by reacting azid with amid in Toluene (C6H5-CH3) at 120 W and 25o for one hour. The yield product was around 70-80%.
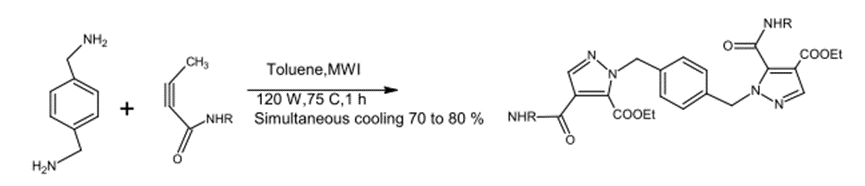
Fig. (4) Shows the reaction of the amide with azide to form heterocyclic compounds.
Diels-Alder reaction generated a yield production of 58-78%
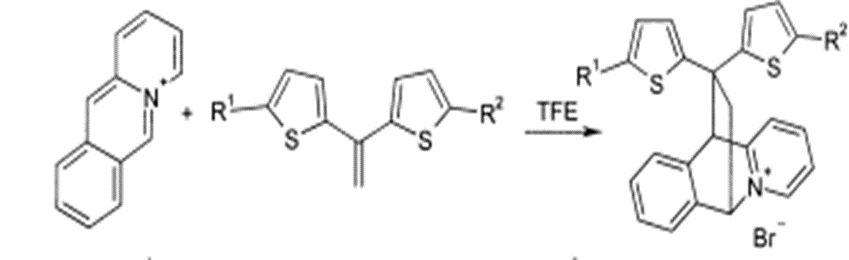
Fig. (5) Represents the Diels-Alder reaction.
4 – Oxidation (22)
Toluene Oxidation with KMnO4 under regular reaction conditions lasts for 10-12 hours compared with such reaction conducted under microwave conditions lasts only for 5 minutes with a yield production of 40%.
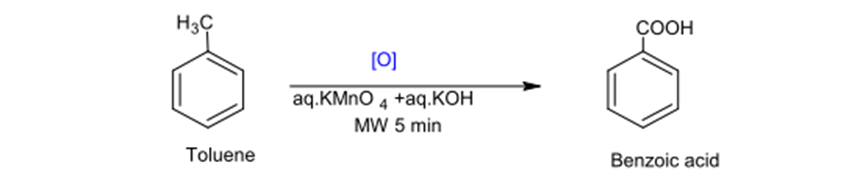
Fig. (6) represents the oxidation of coloring with kMno4 using microwave radiation.
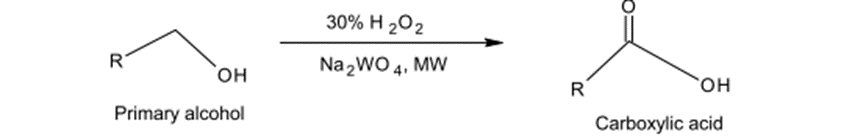
Fig. (7) Represents the oxidation of primary alcohols to carboxylic acid.
5 – Esterification (23)
Prepare propylbenzoate from reacting Benzoic acid with n-propanol which shall be heated in a M.W oven for 6 minutes with the use of concentrated H2SO4 as a catalyst.
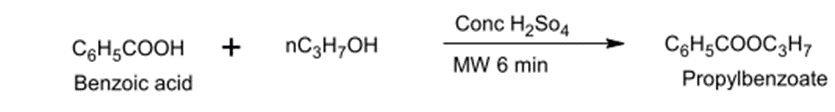
Fig. (8) Shows the reaction of benzoic acid with propanol alcohol.
6- Decarboxylation (24)
The common Decarboxylation process of Carboxylic acids includes the regression of Quinoline with the availability of Copper Chromate (CuCrO4 ). Only a few products are produced out of such a common Decarboxylation process but with the use of Microwave, the Decarboxylation process shall be conducted with a shorter time compared with the common one.
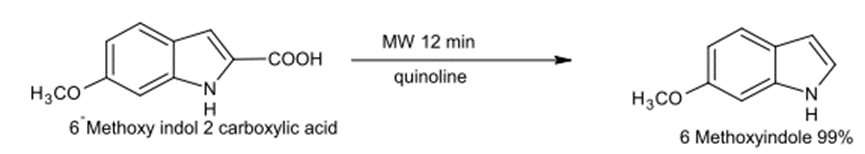
Fig. (9) Represents the removal of the carboxyl group of an amino acid using a microwave.
7 – R – Alkylation (25)
R – Alkylation under microwave radiation with the use of Potassium Carbonate and TBACI generate a product of 65-88%.
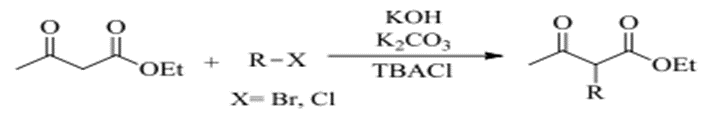
Fig. (10) Represents R-Alkylation using sodium carbonate and TBACI
8 – Schiff’s bases (26)
CO – Amides (R-CO-NH-R) were prepared for Trimethoprim by reacting it with C8H4O3 and C4H2O3 using the common method and the microwave technology with the use of CH3COOH as a solvent. Amides (R-CO-NH-R) for Trimethoprim were transferred into Schiff’s bases through a condensation process in which such Amides (R-CO-NH-R) were reacted with Aldehydes and ketones by using the common method and microwave technology and DMF as a solvent. By using the common method, the reaction lasted for (1-3) hours and 95o-100o.
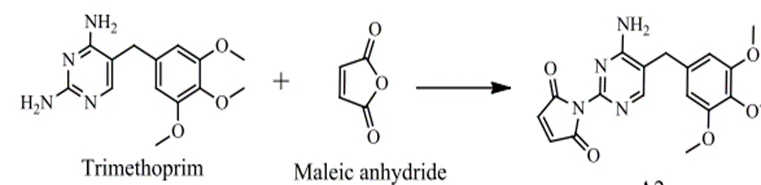
Figure (11) Shows the preparation of trimethoprim monoamide substitutes with maleic anhydride
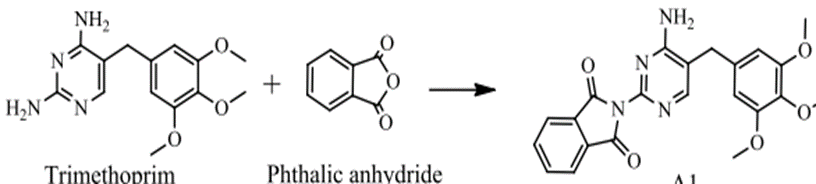
Figure No. (12): Preparation of trimethoprim monoamide substitutes with phthalic anhydride.
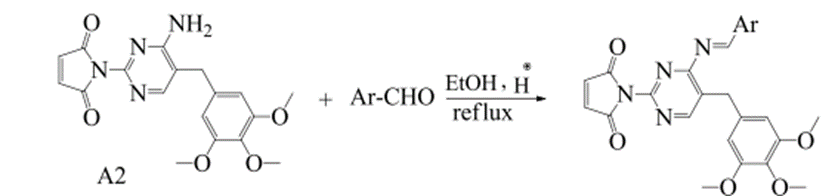
Fig. (13) Shows the preparation of Schiff bases from the reaction of monoimides of trimethoprim with aldehyde.
B –Reaction without the use of solvents (27)
Due to environmental fears and concerns, there has been a growing demand for the satisfaction of solvent–free synthesis processes and reactions. Some old and new approaches are used to reduce the pollution resulting from chemical activities. Therefore, Microwave apparatus has become an important energy source in many lab procedures. Moreover, Reaction without the use of solvents in organic synthesis has been developed as an environmentally friendly process because it includes both selectivity and the invention of a clean environment-based process.
1 – Aromatic Nucleophilic Substitutions (2,1)
was prepared by the reaction made between Sodium Phenoxide and Trichlorotriazine 1,3,5 under microwave radiation for (6) minutes with a yield product of 85-90%
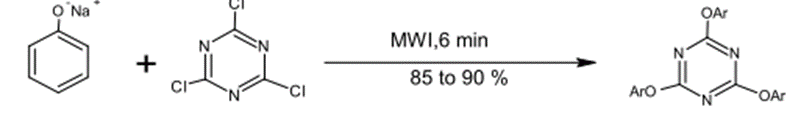
Fig. (14) Represents the substitution reaction with halogens
2 – Deacetylation (12)
Aldehydes, Phenols , alcoholates are protected by acetylation. Deacetylation made under acidic conditions requires long time and yields little product but the use of M.W radiation shortens the time required for Deacetylation and the yield product would be remarkably sufficient.
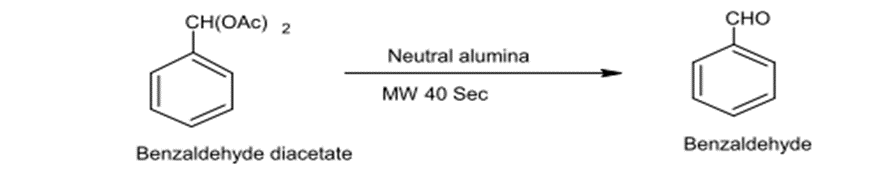
Fig. (15) Represents deacetylation under acidic conditions by microwave irradiation.
3 – Preparation of Polyesters (28)
Polyesters were prepared by exposing solid Carboxylic organic acids (Glycolic acid, Mandelic acid and Benzylic acid) to microwave radiation with 900 W for (2-3) minutes. Such reaction led to the displacement of a water molecule from such acids and the turned to free radicals then to an ester then to polyester.
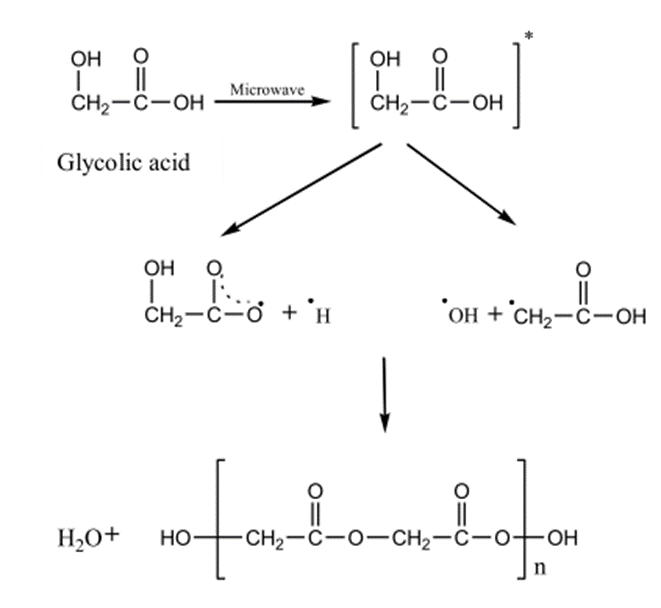
Fig. (16) Shows reaction of glycolic acid to configure polyester.
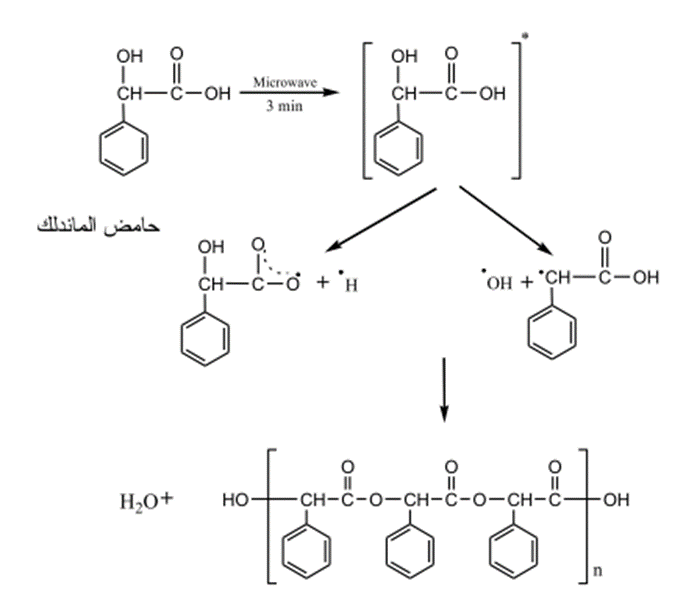
Fig. (17) Shows the reaction of mandelic acid using microwave radiation to form polyester.
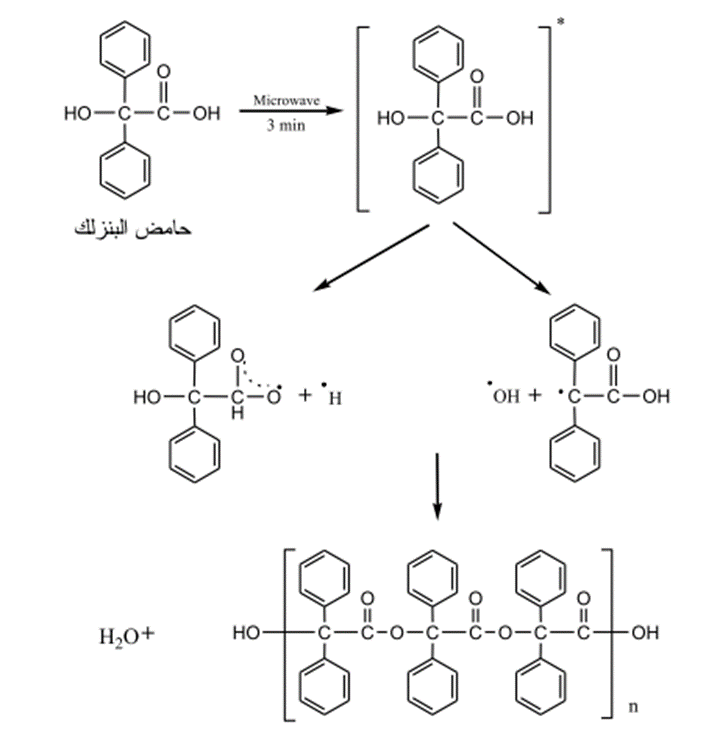
Fig. (18) Shows the effect of microwave radiation on some carboxylic acids containing a
hydroxyl group.
C – M.W-Assisted Reactions Using Solid Liquid Phase
The catalyzing of the solid-liquid phase – transfer is described as an active method used to synthesize organic materials. Such a method is assigned to anionic reactions and it also comprises the anionic activation A catalyzing quantity of tetralkylammonium salt or ion. Equal quantities of a complex factor are added to the mixture for each pure reaction. Such reactions occur in the organic liquid phase.
1) O – Alkylation
Ethereal compounds were prepared from ß- naphthol by using benzyl bromide and methyl-imidazolium tetrafluoroborate-butyl 1-3 under microwave radiation for (6-12) minutes with a yield production of 75-90%. (18)
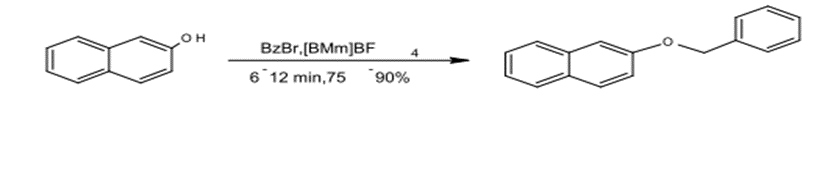
Fig. (19) Represents the preparation of ether compounds from β-naphthol using benzyl bromide with methyl-imidazolium tetrafluoroborate –butyl-3-1 under microwave radiation.
2)N – Alkylation.
Under microwave radiation and with the use of phase-transfer catalysts, N-alkylations occupy a significant space within nonorganic chemistry. Bogdal et al could synthesize N-alkyl phthalimide by using TABA, Phthalimide, Alkyl Halides and Potassium Carbonate. The production yielded a percentage of 45-98%. (29)
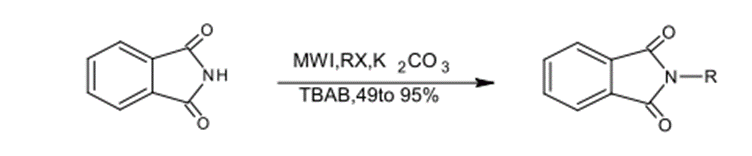
Fig. (20) Represents the synthesis of N-alkyl phthalimide using Na2CO3 with TBAB under microwave radiation.
3 – Oxidations
This method included the oxidation of di alcoholates into Acetone by using PCC, dichloromethane, and tetrabutylammonium bromide. The reaction lasted for (6-8) minutes and the yielded production was around (70-905%). Moreover, Benzyl alcohol was oxidized under microwave radiation for (1-8) minutes to Benzaldehyde (C6H5CHO) and (70-92%) of the product was obtained. (5)
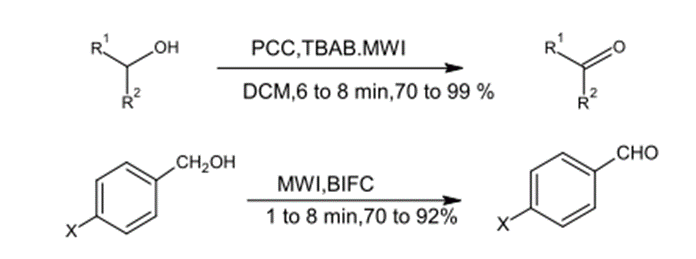
Fig. (21) Represents the oxidation of secondary alcohols to a ketone and the oxidation of benzyl alcohol to benzaldehyde
4 – Knoevenagel Condensation (30)
is a common organic reaction and is applied to synthesize unsaturated acids used in perfumes, Flavonoids and the composition of heterogeneous compounds. Gupta and Wakhloo studied the condensation of Knoevenagel between Carbonyl and Methylene compounds such as Malonic acid. Such condensation process was made with the use of TBAB, Br and K2CO3 in H2O. Unsaturated acids were produced with a proper purity and yielded production.
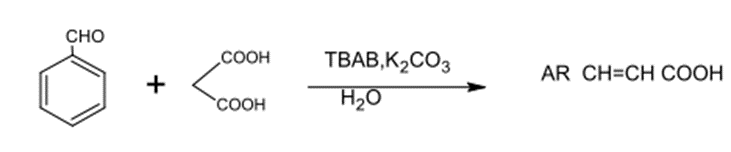
Figure No. (22) Shows the synthesis of unsaturated carboxylic acids from the reaction of the carbonyl compound with the methylene compound, such as malonic acid.
5- Imidazo[1,2-b]pyridazine (32)(33)
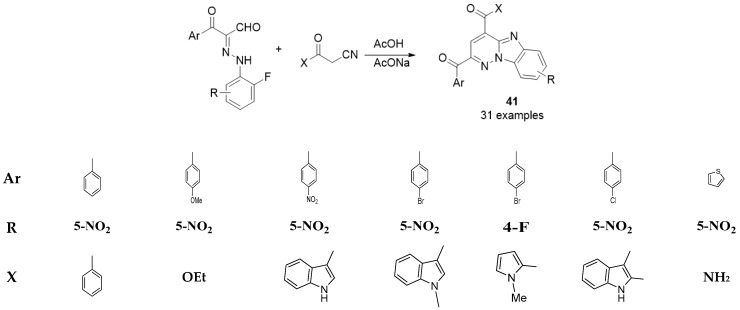
Figure(23)Synthesis of the benzo[4,5]-imidazo[1,2-b]pyridazine derivatives
– Applications
1 – Application in material Chemistry
has been used to synthesize solid nonorganic materials with highly effective and instrumental technology. As to Material Chemistry, Microwave was used to produce ceramic. Ayappa et al (34) conducted a study by placing coal powder in a silica-made crucible which was exposed to microwave radiation for (4-10) minutes inside a M.W oven operated at 2,45 GHz. A significant quantity of SiC was obtained which is widely used in industries such as the manufacturing of grinding wheels and skimming equipment.
2 – Preparation of catalyst under microwave irradiation
A high permeability membrane of NaA zelotie YB2 Cu3O7-x (35) was prepared by reacting Alominates with Na2O3Si in proportions a molarity percentage in an oven operated with 2450 MHz for (15) minutes. The Zeolite membrane which was synthesized by microwave heating appeared to be four times higher than the one synthesized by traditional heating.
3 – Medicinal Application
Microwave radiation has been recently subject to a wide range of studies and researches to indicate its effect on biological systems. Rany(51) studied the effect of microwave radiation on blood protein albumin and gluten protein. Many other researches have been conducted (36) (37) to prepare polymer (Lactic acid – glycolic acid) and to study its physical characteristics and their biological effects due to its various medical usages and medicine development. Such microwave radiation is used in(38)
- Testing medicine formulas synthesized out of organic compounds.
- The composition of long chains of peptides.
- Magnifying DNA to facilitate its usage in disease analysis (39).
- The synthesis of radiative pharmaceutical treatments including short half-life isotopes through the catalyzing via the use of Microwave energy. (40)
- The synthesis of Nanomaterials.
- Free-solvent reactions. (41)
- The synthesis of isotopes.
- The composition of Polymer chains.(42)
- Peptides composition. (43)
- Medical and green chemistry ( 44)
Conclusions:
Microwaves represent an alternative energy source for chemical reactions and processes. Through dielectric heating, reaction mixtures are heated homogeneously without contact with walls. Reaction times are significantly reduced compared to conventionally heated systems (heat) while maintaining acceptable percentage yield and selectivity. A minor drawback is that chemical reactions and processes in the microwave field are more dependent on the tools and materials used than in the case of heat heating.
References
1- Seijas, JA. Vazquez-Tato, MP . Martinez, MM. Corredoira, GN.(1999) J. Chem. Res.(S); 1999; 420-42
Ganzler,K. Szinai,I. Salgó,A .( 1990) J. Chromatogr 520, 257-262 2-
3- Katia Martina ,Giancarla and Rajender S.Vama (2021) Impact of Microwaves on Organic Synthesis and Strategies toward Flow Processes and Scaling Up
J.org.chm1. 3857-13872
4-Sangita Dayanand Katre(2023) Microwaves in Organic Synthetic Chemistry- A Greener Approach to Environmental Protection: An Overview Asian Journal of Green Chemistry 8 68-80
5-Vass, A. Dudas, J. Varma, RS.(1999) Tetrahedron Lett( 40) 4951–4954.(b) L Perreux; A
6- V Chakraborty; M Bordoloi; J. Chem. Res. (S); 1999;118–122
7- Vladimoar C. and Milan H. (1999) Journal of Photochemistry and Photobiology A: Chemistry., 123: 21-23
8- Wei X.-L. Shi Q.-L. Jiang L. Qin Y. New J. Chem. 2023;47:15525–15533.
9- Pei K. Li D. Qi W. Wu D. Molecules. 2023;28:1892.
10- Adhikari A. Bhakta S. Ghosh T. Tetrahedron. 2022;126:133085.
11- Zhu F. Lu H. Lu Y. J. Mater. Sci. 2023;58:4399–4415.
12-Martinez V. Stolar T. Karadeniz B. Brekalo I. Užarević K. Nat. Rev. Chem. 2023;7:51– 65 .13- Scharn, D . Wenschuh, H. Reineke, U. Schneider-Mergener, L. Germeroth, J.( 2000) . Comb. Chem( 2) 361–369.
14- Sekhon BS.(2010) Microwave-Assisted Pharmaceutical Synthesis: An Overview, Int J PharmTech Res 2(1): 827-833 DOI: 10.4236/oalib.1100686
15- Breslow.R(1991) Acc. Chem. Res( 24) 159–164.
16– Grieco, PA. Brandes, EB. McCann, S. Clark, JD.(1989) J. Org.Chem( 54) 5849–5851
17- Martelanc, M. Kranjc, K . Polanc, S. Koevar, M.(2005) Green Chem.( 7) 737–741
18– Hren, J. Kranjc, K. Polanc, S. Koevar, M.(2008). Synthesis. 452–458
19- Elisabetta C. Donatella G. Sofia A. Caterina F. Manuela S. Dorotea
20- Gedye, R N. Rank, W Westaway, KC. (1991)Can J. Chem. 69-700
21- Katritzky, AR. Zhang, Y. Singh, SK ,(2003) SteelArkivoc ;xv; 47–64 16- Gedye, MN. Smith, FE. Westaway, KC. (1988) Can J. Chem, 66,17
22- Gedye, RN. Smith, E. Westaway, K. Ali Baldisera, H. Laberge, L. Rousell, J . (1986) Tetrahedron let;27;279;
23- Jones, GB. Chapman, BJ(1993). J. org chem. ;58; 5558.
24- Chakraborty, V. Bordoloi, M. ( 1999) J. Chem. Res.(S) ,118-122
25- Lee, KY . Case, ED. Asmussen, J .(1997) J Mater. Res. Innovat(1)101
26- Bogda D ; Pielichowski J ; Borona A ; Synlett; 1996; 873–8
Synthesis, Eds., BlackwellOxford,. (b) Lidstrom, P. Tierney, J. Wathey, B. Westman, J.(2001) Tetrahedron 57;9225-9283
27- Gupta, M. Wakhloo, BP.(2007) Arkivoc; (i); 94–98.
28-Polshettiwar V, Nadagouda M N, Varma R S, MW-assisted chemistry: a rapid and sustainable route for synthesis of organics and nanomaterials Aust. J. Chem., 2009, 62, 16-26
29- Motshekga S C, Pillai S K, Ray S S, Jalama K, Krause R W M, Recent Trends in the Microwave-Assisted Synthesis of Metal Oxide Nanoparticles Supported on Carbon Nanotubes and Their Applications, Journal of Nanomaterials Volume 2012, Article ID 691503, 15 pages, doi:10.1155/2012/691503
30- Singh D, Rawat D and Isha (2016) Microwave-assisted synthesis of silver nanoparticles from Origanum majorana and Citrus sinensis leaf and their antibacterial activity: a green chemistry approach. Bioresour. Bioprocess. 3: 14 DOI 10.1186/s40643-016-0090-z
31- Hasanpoor M, Nabavi H F and Aliofkhazraei M(2017), Microwave-assisted Synthesis of Alumina Nanoparticles Using Some Plants Extracts J Nanostruct, , 7(1): 40-46
32-Garrido A., Vera G., Delaye P.O., Enguehard-Gueiffier C. Imidazo(2021) [1, 2-b] pyridazine as privileged scaffold in medicinal chemistry: An extensive review. Eur. J. Med. Chem. ;226:113867.
32- Javahershenas R. Nikzat S. RSC Adv. 2023;2023:16619–16629.
33- Vanhoof J. R. Dirix R. De Vos D. E. Green Chem. 2023;25:978–985
34- http://www.pharmaceutical-technology.com
35- John R. Jones J. R., Lu S.(2002), Microwave-enhanced radiochemistry. Microwaves in organic synthesis. Wiley-VCH Verlag GmbH & Co. KGaApp, 435-462
36- Elisabetta C. Donatella G. Sofia A. Caterina F. Manuela S. Dorotea M. Maria G. Rosario P. and Francesco C.(2008) Biomaterials., ( 29) 1400-1411
37- Santagada,p . Frecentese F, V. Perissutti E. Fiorino F., Severino B., Caliendo G.(2009) Microwave assisted synthesis: a new technology in drug discovery. Mini Rev. Med. Chem., 9: 340-358
38- Santagada,p . Frecentese F, V. Perissutti E. Fiorino F., Severino B., Caliendo G.(2009) Microwave assisted synthesis: a new technology in drug discovery. Mini Rev. Med. Chem., 9: 340-358
39- Fouad, M. A.; Abdel-Hamid, H.; Ayoup, M. S. Two decades of
recent advances of Ugi reactions: synthetic and pharmaceutical
applications. RSC Adv. 2020, 10, 42644.
40-. V. Polshettiwar, R. S Varma, (2008) Aqueous microwave chemistry: A clean and green synthetic tool for rapid drug discovery, Chemical Society Reviews, 37: 1546-1557. 41- A. Stadler, (2002) Microwave-enhanced reactions under open and closed vessel conditions: A case study, Tetrahedron, 58: 3177-3183
42- R. S. Varma, (2006) Greener organic syntheses under non-traditional conditions, Indian Journal of Chemistry, 45B, 2305-2307
43- A Seijas, M. P. Vazquez-Tato, (2007) Microwaves: A new tool for an ancient element. solvent and support-free microwave-assisted organic synthesis , Chimica Oggi, 25: 20-26
44- Fairoosa, J.; Saranya, S.; Radhika, S.; Anilkumar, G. Recent Advances in Microwave Assisted Multicomponent Reactions. Chem. Select 2020, 5, 5180.
