Evaluation of Groundwater Resources and Their Suitability for Drinking and Irrigation Purposes in Al-Marj City, NE Libya
Saleh A. Albbanqeeyah1*, Gabril Elshlmani2, Khalefa Mosbah3, Jadlmulah Masoud4, Fares F. Fares5
1* Department of Water Technology in The Higher Institute for Agricultural Techniques EL- Marj – Libya. (albangea@yahoo.com)
2. Faculty of Environmental Sciences, University of Benghazi, Al-Marj Branch- Libya.
3. Department of Zoology, Faculty of Science, Ajdabiya University, Libya.
4• Department of Water Technology in The Higher Institute of Science and Technology Tazerbo – Libya.
5 Department of Earth Science, Benghazi University, Libya
HNSJ, 2024, 5(12); https://doi.org/10.53796/hnsj512/22
Published at 01/12/2024 Accepted at 05/11/2024
Citation Methods
Abstract
Groundwater resources in the AL-Marj area were evaluated for suitability for drinking and irrigation use by determining the main physical and chemical properties in 2023. pH, conductivity, total dissolved solids, total hardness, turbidity, major cations (Ca+2, Mg+2, Na+, and K+), major anions (HCO–3, Cl–, NO–3, and SO4-2), and heavy metals (Fe+2, Cu+2, Zn+2, Pb+2, and Cd+2) were all measured in a several of samples taken from 9 various wells. The findings revealed significant differences in the physical and chemical characteristics of the analyzed samples. However, the majority of values fell short of the WHO drinking water standards’ maximum possible levels. According to the quality assessment, the study area’s groundwater is generally not completely suitable for direct drinking in terms of TDS, EC, Cd+2, and Pb+2. Based on the sodium ratio and EC, the water in the wells under study is suitable for irrigational purposes. In addition, the general chemistry of water samples was prevailing mainly calcium bicarbonate..
Key Words: Groundwater. Heavy metals. Water. Resources. AL-Marj area.
Water is essential for humans, animals, plants, agriculture, energy production, and industrial processes (Department of the Environment. 1992). About 97 percent of the world’s freshwater is underground, and it is the primary source of rivers and lakes (Baird & Cann 2011). Groundwater is water found beneath the earth’s surface in pores and fractures of soil and rocks (Goulburn-Murray Water. 2015). Groundwater is an unavoidable water resource for domestic and drinking purposes in urban Africa (MacDonald et al., 2011). Groundwater chemistry is heavily influenced by geochemical processes, regional geology, and land use patterns (Matthess, 1982; Kumar et al., 2006; Liu et al., 2008; Zhu and Schwartz, 2011; Rajesh et al., 2012). Libya’s water comes from four sources: groundwater, which supplies nearly 95 percent of the country’s needs; surface water, which includes rainwater and dam construction; desalinated seawater; and wastewater recycling (Wheida,2007). Therefore, can determine the quality of water by examining its physical, chemical, and biological properties. Water is being polluted more than ever before, consequently of increased population, industrialization, the use of fertilizer in farming, and man-made actions. Groundwater is the primary source of water for domestic and drinking purposes in many developing countries (Midhun Dominic & Shino Chacko 2016). In addition, Agriculture is the main source of income for the residents of Al-Marj, and they rely heavily on groundwater for drinking, domestic, livestock, and agricultural purposes (Nair, G.A et al 2006). As a result, estimating groundwater quality is critical for determining hygienic and appropriate groundwater sources. The human population in many parts of the world is facing critical water supply and contamination issues. African countries face challenges in obtaining safe drinking water and adequate sanitation (Tuinhof, A.; al el 2011). Groundwater contamination is especially severe in arid or semi-arid regions where water supplies are scarce (Hamzaoui-Azaza, F, al el 2012). Due to high concentrations of certain chemical and physical parameters, groundwater quality in Libya is a critical issue that threatens human health (Rashrash, al el 2015). Many researchers have assessed groundwater to their suitability for drinking in various parts of Libya, and some of them have found that, tested elements were within the standard limits, Achuthan al el (2006) indicated that water at all places was within the standard limits when they evaluated groundwater quality of north-east Libya. Whereas Elgali (2015) had found the groundwater clean in only 14.3% of the groundwater samples in Derna city. In addition, Albanqeeyah (2021) had motioned that the concentration of nitrates in the groundwater in Awjilah was higher than the standard limits, while it was within the standard limits in Jabal Al Akhdar region. Moreover, Roshrash, al el, (2015) in their study found a significant rise in the majority of water ions, such as dissolved salts, Nitrates, sodium, and chlorides. It might be attributed to either the disposal of untreated wastewater from disposal ponds and septic tanks or the infiltration of irrigation water. According to the Megahed al el, (2021) the current quality groundwater in Al Jabal Al Akhdar, except for a few samples classified as unfit, groundwater is rated as excellent and doubtful. Therefore, the primary aim of this work is to evaluate groundwater quality by testing 9 groundwater wells in the study area using physicochemical parameters.
1.1Location map of the study area
The research was conducted in the Libyan city of Al-Marj. The city is in the north-eastern part of Libya, on the Mediterranean Sea’s coast. It has a land area of about 10,000 km2. The Mediterranean climate, with hot, dry summers and mild, rainy winters, has an impact on the study area. moreover, it has a dry climate, similar to that of a semidesert, with minimal rainfall and high evaporation rates, as well as a distinct appearance of aridity that prevails throughout the entire area. Al-Marj is located on the Cyrenaica level on the western edge of Jebel Akhdar and has a population of 85,315 people as of the beginning of 2012 with coordinates 32_29012” N 20_50002” E. The precise locations of the sampling points of the groundwater wells were determined in the field using (Jaouda al el, 2017). In addition, Soils of El-Marj was humus rich clayed carbonate mixture called ‘Rendzinas’. The soils of it was red color. It belonged to intrazonal hiolithogenic group of soil that was alkaline with a total nitrogen range from 0.04% to 0.20%; 1.35% clayed residues and 1.34% calcium carbonate. It was a red rock composed of clayed limestone residues that formed soil. It was alkaline, containing high levels of calcium, magnesium, and iron and low levels of nitrogen (0–13%). The amount of organic matter in the surface soil was approximately 1.67%, but as depth increased, this percentage dropped (Nair et al., 1996).
|
Number |
Location |
Altitude |
Longitude |
|
1 |
Residential |
32.496501 |
20.859535 |
|
2 |
Residential |
32.496324 |
20.862006 |
|
3 |
Residential |
32.497848 |
20.864204 |
|
4 |
Residential |
32.508477 |
20.861527 |
|
5 |
Farm |
32.503601 |
20.862879 |
|
6 |
The edge of town |
32.5032 |
20.856189 |
|
7 |
The edge of town |
32.501007 |
20.853749 |
|
8 |
The edge of town |
32.500281 |
20.830645 |
|
9 |
Residential |
32.483835 |
20.841003 |
Table 1 shows the Main wells with their coordinate, altitudes and names in the study area.
Figure 1 shows the groundwater wells (water sampling) in study area at Al-Marj city in northeast Libya.
1.2. Aquifer of Al -Marje City
The geological and hydrogeological studies (e.g. Ali, 2009) show that there are three horizons in Al Marj plain, these horizons are:
The first horizon: These bodies of ground water perched on clay or shale beds occur locally of Pleistocene age. The water in this horizon is characterized by high salinity (Figure 2).
The second horizon: The water in this horizon found in clayey sandy and gravel layer, the depth of this horizon is ranged between 25 and 90 m, the salinity of the water is increased as we move towards the middle of the plain, this horizon of Pliocene age. The available amount water in this layer is very limited.
The third horizon: Bodies of ground water of this horizon found in limestone reservoir of Eocene age. This horizon is considered to be the most important horizon of the groundwater in the plain in term of the water quality and availability. The depth of this horizon is between 150 and 500 meters (Figure 2).
Figure 2 Subsurface map showing the main aquifers in the study area (Al Awami, 2002)
2. Materials and Methods
2.1. Sample Collection and Analysis
Water samples were collected from different 9 wells shown in figure (1) in study area. It can be seen that, the groundwater wells (sampling points) in the study area in Al-Marj city in northeast Libya in table (1). The coordinates of the groundwater wells were determined using the Global Positioning System (GPS). This was done during the middle of the wet season on 1 June 2023. First, water samples were collected in clean glass bottles with a minimum capacity of 250ml. A water pump was used to collect groundwater samples. Second, the date and source of the water sample were labeled on all bottles. Water samples were then stored in an icebox at 4 °C to avoid changes in chemical parameters caused by photochemical reactions. The physical and chemical parameters of the water samples were performed using standard equipment and materials, in the water analysis laboratories.
Total Dissolved Solids (TDS), pH, and electrical conductivity (EC) were measured using a Denver Instrument Model 50. A LaMotte turbidimeter (Model: 2020 we/wi, LaMotte Company, Chestertown, MD, USA) was calibrated by using formazin standards to measure the turbidity of the water samples. HCO3 and Cl– were determined using titration techniques, while SO4 was determined using gravimetric methods. Whereas NO-3 and Iron (Fe–) were analyzed by 100 Dionex Ion Chromatography instrument equipped with AG4A-SC guard column, AS4ASC separating column, SSR1 anion self-regeneration. Suppresser and conductivity detector. Used the AAS Hitachi-5000 to measure Ca, Mg, Na, P, and K. In addition, the atomic Absorption method model (Perkin Elmer, model 2380) is used to calculate heavy chemical metal concentrations such as; Zinc (Zn), Copper (Cu), Cadmium (Cd), and Lead (Pb).
3. Results and discussion
Major ions like these are included in the chemical analysis of the water samples under study Na+, Ca+2, Mg+2, K+, Cl–, HCO-3, NO-3, and SO4-2 and heavy metals for instance Zn, Cu, Cd, Fe, and Pb tale (2) were collected from different 9 wells in AL-Marj city and analyzed for several chemical and physical parameters shown in Table 2.
Table 2 gives the statistical analysis of the physiochemical parameters of groundwater in the region of Al-Marj city, east Libya.
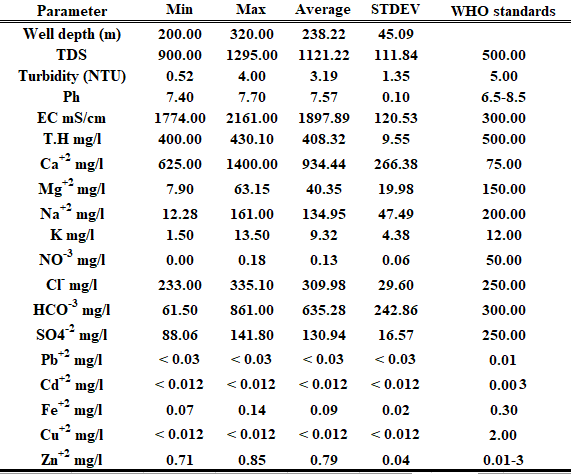
Table 2 Statistical summary of physiochemical characteristics of groundwater
TDS and EC are considered the most important factors when assessing water quality, the TDS concentration is proportional to the measured EC parameter in water samples with a proportional constant that varies between 0.55 and 0.7(Hem D 1985. Brown E al el,1960). The minimum and maximum values of the TDS concentrations for groundwater are (900 – 1295 mg/l). Meanwhile, the corresponding EC measurements are (1774 – 1295 mS/cm) as shown in figure 3, all TDS and EC values of the groundwater fall over the standard value recommended by WHO. In addition, due to the complex geomorphological makeup of the study area, this ratio increased as pH levels decreased, indicating a greater release of inorganic matter. Moreover, with a maximum level of 7.70, the field measurements showed basic pH levels for groundwater. This suggests that the amount of bicarbonate in the water is high. However, all measured pH values are within the permissible limits suggested by WHO drinking water standards.
Figure 3 shows the concentration of TDS and EC
The TH concentrations in water samples that were measured showed similar trends in terms of alters in EC and TDS. The amount of heavy rain and leachate drainage from agricultural fields and farming, as well as the use of well water for gardening and agriculture, all impacted the TH concentration in groundwater. However, it ranged from 400 to 430 mg/l. Calcium and magnesium ions are the main contributors to total hardness (TH) (Fetter 1988). A previous study by James, M, (2017), evaluated the groundwater wells in the Liberian city of Paynesville; the findings indicated that the TH distribution ranged from 25 to 425 mg/L.
The study area’s groundwater wells had turbidity levels ranging from 0.52 to 4 nephelometric turbidity units (NTU), as indicated in Table 2. Five groundwater wells had the highest amount of turbidity (4 NTU), while groundwater well number 4 had the lowest level (0.52 NTU). However, the sewage discharge from the residential area that made its way to the groundwater well and the soil erosion brought on by human activity and natural phenomena close to the study area were the causes of the high turbidity value. In addition, the turbidity value in the current study stayed within the WHO drinking water guidelines (5 NTU).
The minerals gypsum, aragonite, anorthite, calcite, and dolomite are examples of geological sources of calcium variables (Macdonald 1965; Bender 1974). The calcium content of the groundwater samples varied from 625 to 1400 mg/l with an average of 934.33 mg/l. It is primarily ascribed to the infiltration of rainwater, which releases calcium into the groundwater from sedimentary carbonate rocks and soil aquifer materials (Magaritz et al. 1989). Sodium concentrations in the collected water samples ranged from 12.28 to 161 mg/l with average 134.95 mg/l. Strong water–aquifer interaction brought on by cation exchange and human activities like wastewater disposal may be indicated by a high concentration of Na in the groundwater wells. Water naturally contains sodium ions as a result of several processes, including weathering of clay, vaporization, and agricultural and human activities (Siemens, al el 2020).
Sulfate values in groundwater samples varied from 88.06 to 141.8 mg/l, with a mean of 130.94 mg/l. Well, no. 6 presented the highest value of sulfate, while well no. 8 presented the lowest value. Sulfate ions were found in water samples as a result of gypsum dissolution, rock weathering, and household wastewater (Fetter 1988). Bicarbonate concentrations in water samples ranged from 61.5 mg/L in well no 8 to 861 mg/L in well no 1 as shown in Table 2. Nearly 88.8 % of the analyzed samples exceeded the standard limit of 300mg/L recommended by WHO whereas approximately 11.1% of samples were below this limit. According to table 2, chloride concentration in the groundwater wells varied from 233 mg/L to 335.1 mg/L. Groundwater well number 8 had the lowest value, and groundwater well number 6 had the highest level. The chloride concentration in only one groundwater well was below the WHO-recommended drinking water limit. However, the overuse of groundwater may be the cause of this rise.
Figure 4 shows the concentration of T.H, Ca+2, Na+, Cl–, HCO–3, and SO4-2
As shown in the table 2 and figure 5, the potassium content of the evaluated groundwater wells varied from 1.50 to 13.5 mg/L. However, approximately 22.2 % of the analyzed samples exceeded the standard limit of 12 mg/L recommended by WHO whereas 77.7% of samples were below this limit. Wastewater in the study area may be the primary source of the increasing amount of pollutants, including potassium, in the groundwater wells. In addition, to solid and liquid wastes, rock weathering may be the main source of potassium. In the investigated subterranean wells, the potassium content was within the WHO-recommended drinking water limit 12 mg/l. Jauda al el (2021), assessed the quality of ground water in AL-Marj city and discovered that the potassium value ranged from 2.3 and 12 mg/L.
The dissolution of all solids and rocks, but primarily of limestone, dolomite, and gypsum found in significant amounts in certain brines was referred to as the abundance of magnesium in the groundwater wells. The magnesium concentration in the studied groundwater wells varied from 7.9 mg/L to 63.15 mg/L with an average 40.35, as shown in table 2.
Figure 5 shows the concentration of Mg+2, and K+
The finding obtained from Table 2 shows the nitrate concentration of groundwater obtained within the period of study between 0.0 and 0.18 mg/l with an average of 0.06 mg/l. Comparing the obtained value of nitrate with the standard of 50 mg/l (WHO, 2022) shows that all obtained values fall lower than the allowable maximum permissible for drinking water. Albanqeeyah, (2021) determined the nitrate concentration of groundwater in the AL-JABAL AL-AKHDAR district of Libya and observed that the concentration of NO–3 in the groundwater wells varied between 0.7 and 13.1 mg/L. Figure 6 shows an inverse relationship between groundwater nitrate concentration and depth, whenever the depth rises, whenever concentration decreases.
Figure 6 shows relationship between groundwater nitrate concentration and depth
This slight increase in nitrate concentration particularly in shallow wells compared with deep wells might be attributed to using fertilizers in irrigation and /or infiltration of domestic wastewater from cesspools distributed within the study area.
According to their values, heavy metals in the natural surface and groundwater are typically divided into major, minor, and trace levels. Due to human activity or natural processes, these metals may enter groundwater. The concentrations of heavy metals in water are primarily caused by two natural processes: chemical weathering and soil leaching (Drever 1988). Table 2 displays the findings of the heavy metal analysis. In addition, the release of heavy metals from primary materials and soil is influenced by several factors, including pH, adsorption, hydration, and co-precipitation. These factors further impact the stability of trace metals in water (Drever 1988; Fetter 1988). The findings revealed that all heavy metals, except Pb+2 and Cd, are within the WHO-recommended drinking water limits table 2. Furthermore, lead and cadmium contamination of groundwater is indicated by their concentrations measured from various wells that are less than (<0.03 and <0.012 mg/L), respectively, but were marginally higher than the WHO-recommended permissible limit of (0.01 and 0.003) mg/L consecutively. The significant levels of lead and cadmium suggest the impact of nearby human-driven activities, including farming in AL-Marj city and other ventures around groundwater wells, on the water’s composition. The concentration of iron, copper, and zinc in water samples were below the permissible limits for drinking water use.
Overall, the chemical analysis’s findings demonstrated that both natural and man-made activities significantly impact the groundwater’s chemical composition in the study area. This is consistent with other researchers’ findings for various locations (Shi et al. 2012; Vousoughi et al. 2013).
3.1. Classification of groundwater according to A piper diagram
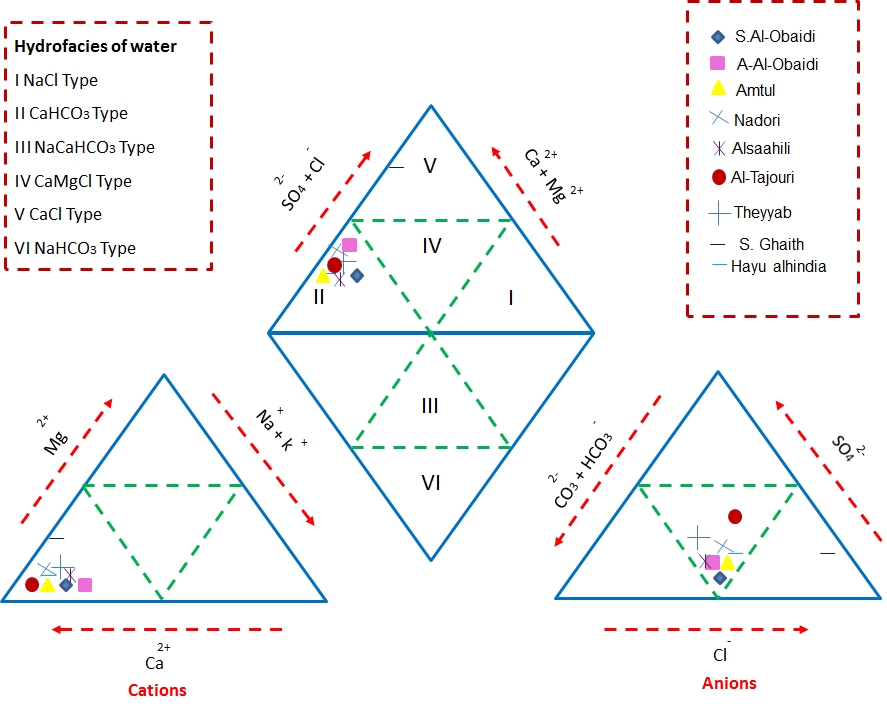 A Piper (Piper 1953) diagram of Cations Na, K, Ca, and Mg and Anions HCO-3, Cl, CO3 a, and SO4 was used to determine water types for the studied areas Figure 7. The Piper diagram revealed the groundwater in all wells are characterized by CaHCO3 type II except S. Ghaith well (well no 8) is characterized by CaCl type V.
A Piper (Piper 1953) diagram of Cations Na, K, Ca, and Mg and Anions HCO-3, Cl, CO3 a, and SO4 was used to determine water types for the studied areas Figure 7. The Piper diagram revealed the groundwater in all wells are characterized by CaHCO3 type II except S. Ghaith well (well no 8) is characterized by CaCl type V.
Figure 7 Piper diagram of water chemistry in the study area (fields after Tweed et al., 2005).
3.2. Classification of water based on Total Dissolved Solids
The values of TDS in the studied wells range from 900 to 1295 mg/l with a mean of 1112 mg/l, the WHO (2022) drinking water guideline shall not be more than 500 ppm. All the groundwater samples were above the permissible limit. According to TDS classification table 3, the studied water samples were classified as brackish water and useful for irrigation table 4.
Table 3 Classification of water based on Total Dissolved Solids (after Fetter,1994).
Table 4 Classification of water based on Total Dissolved Solids (after Davis and De Wiest, 1966).
The discrimination diagrams based on Cl /Cl+HCO3 vs. TDS indicate the dominance of rock in the studied well figure 8.
Figure 8 Dominance of precipitation, rock and evaporation on Na/Na+Ca vs. TDS of the study area (fields after Gibbs, 1970).
The plot of Mg/Ca vs. Na/Ca showed that the source of ions in the studied samples mainly originated from limestone figure 9
figure 9 Plot of Mg/Ca vs. Na/Ca ratios of the studied water samples (modified after Han and Liu, 2004).
3.3. Irrigation water quality
Groundwater quality was assessed in this study to ascertain its suitability for irrigation by identifying the primary parameters that represent its quality, such as EC and sodium hazards. In addition, since EC reflects the TDS in water, it is a useful indicator of salinity. Excessive EC, or water salinity, puts crops at risk by decreasing plant osmotic activity, which hinders the plant’s ability to absorb water and nutrients from the soil (Saleh et al. 1999; Subramani et al. 2005). However, in this study, electrical conductivity (EC) averages 1897 mS/cm, and is classified as class 3, fair quality shown in table 5.
Table 5 Types of groundwater according to EC (US Salinity Laboratory ,1954)
Results of Na % for all samples were less than 40 % and according to Plot classification, the waterfalls in the field were excellent category figure 10 suggesting that the water is suitable for irrigation use.
figure 10 Plot of EC vs. Na % showing the classification of irrigation water (fields after Johnson and Zhang, 1990).
4. Conclusion
The physical and chemical composition of groundwater at the AL-Marj basin was investigated throughout the study period in 2023, representing the study of elements and knowledge of water quality in the study area. The physical and chemical composition of groundwater samples showed that the EC of the measured groundwater wells was noticeably high. All groundwater wells presented high concentrations of TDS, calcium, and bicarbonate, and one sample had a value lower than the WHO standard. In addition, the primary causes of salinity in the groundwater samples were bicarbonate, calcium, chloride, and sulfate. According to the quality assessment, the groundwater in the study area is generally not completely suitable for direct drinking in terms of EC, TDS, Pb, and Cd. Moreover, according to the sodium ratio and conductivity values, the water sources examined can be used for irrigational purposes. Furthermore, the Piper diagram showed that, except well number 8, all wells’ groundwater is classified as CaHCO3 type II. According to this study, groundwater in the study area needs to be monitored and protected from pollutants, whether from agricultural practices or wastewater released from cesspools.
References
Al Awami, Y. (2002): Planning and development of the water field, SW Al Marj. Technical report. In Arabic.
Albanqeeyah, Saleh., (2021). A comparative study of nitrate ion concentration in groundwater basins in Libya a case study between AL-JABAL AL-Akhdar and the Awjilah oasis, Cyrenaica, Libya. Humanitarian & Natural Sciences Journal, volume 2. Issue1, P (408).
Baird, C.; Cann, M. 2011. Química Ambiental. 4nd ed. Brookman, Porto Alegre, 844p.
Bender F (1974) Geology of Jordan. Supplementary edition in English with minor revision. Berlin, Germany.
Brown E, Skougstad M and Fishman M. Methods for collection and analysis of water samples. Geological Survey Water-Supply Paper, 1960, 1454: 310.
Davis, S.N. and De Wiest, R.J. (1966): Hydrogeology, Vol. 463, Wiley, New York.
Department of the Environment (1992). Using water wisely. Department of the Environment Welsh Office.
Drever JF (1988) The chemistry of natural waters, vol 3. Prentice- Hall, New York.
Fetter CW (1988) Applied Hydrogeology, vol 2. Merrill publishing company, London.
Gibbs, R.J. (1970): Mechanisms controlling world water chemistry. Science; 170: 1088-1090.
Hamad, J. R. J,Yaacob, W.Z.,& Omran, A. (2021). Quality assessment of groundwater resources in the city of Al-Marj, Libya. processes,9(1), 154.
Hem D. Study and Interpretation the Chemical of Natural of Characteristics Natural Water, 3rd edition USGS Water-Supply Paper 2254 66-69 US Govt Printing Office Washington DC., 1985.
Hamzaoui-Azaza, F.; Ameur, M.; Bouhlila, R.; Gueddari, M. Geochemical characterization of groundwater in a Miocene Aquifer, Southeastern Tunisia. Environ. Eng. Geosci. 2012, 18, 159–174
Han,G. and Liu, C. (2004): Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China Chemical Geology; 204: 1-21.
James, M. Spatial analysis of Groundwater sources in the duport road (shara and cow field) and soul clinic diamond creek communities, Paynesville City, Republic of Liberia. IJSRST 2017, 3, 1–12.
Jaouda, J.R.H.; Hanafiah, M.M.; Abdullah, S. Problems and current practices of solid waste management in the city of Al-marj, Libya. J. Clean WAS 2017, 1, 1–5.
Johnson, G. and Zhang, H. (1990): Classification of Irrigation Water Quality, Oklahoma cooperative extension fact sheets (available at http://www.osuextra.com).
Khaled Megahed. Maie El-Gammal. Mahmoud Ibrahim. 2021. quality assessment of the surface and underground water in the region of Al Jabal Al Akhdar, Libya. Health and Environment Journal, volume 2. Issue1, p (119).
Liu, C.W., Jang, C.S., Chen, C.P., Lin, C.N., Lou, K.L., 2008. Characterization of groundwater quality in Kinmen Island using multivariate analysis and geochemical modelling. Hydrol. Processes 22, 376–383.
Macdonald M (1965) Hydrogeological survey of the Madaba-Ma’an area, Jordan. vol 2, pp. 30 Report at the Water Authority of Jordan, Amman. Jordan.
Matthess, G., 1982. The Properties of Groundwater. Wiley, New York (p. 498).
Kumar, M., Ramanathan, A.L., Rao, M.S., Kumar, B., 2006.Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. J. Environ. Geol. 50, 1025–1039.
Magaritz M, Aravena R, Pena H, Suzuki O, Grill A (1989) Water chemistry and isotope study of streams and springs in northern Chile. J Hydrol 108:323–341.
Midhun Dominic, C. D. & Shino Chacko, T. T. 2016 Analysis of water quality of samples collected from Thevara Region, Kerala, India. International Journal for Research in Applied Science and Engineering Technology 4, 382–388.
Nair, G.A.; Bohjuari, J.A.; Al-Mariami, M.A.; Attia, F.A.; El-Toumi, F.F. Groundwater quality of north-east Libya. J. Environ. Biol. 2006, 27, 695–700.
Nair, G.A., F.F. El-Toumi, K.M.A. El-Tayeb, A.M. Bosnaina and K.C. Bhuyan: Habitat, occurrence and density of some pulmonated slugs of north-east Libya (Mollusca, Milacidae and Limacidae). J. Afr. Zool., 110, 251-256 (1996).
Rajesh, R., Brindha, K., Murugan, R., Elango, L., 2012. Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environ. Earth Sci. 65, 1203–1213.
Rashrash, S.M.; Ben Ghawar, B.M.; Hweesh, A.M. Evaluating groundwater pollution using hydrochemical data: Case study (AlWahat Area East of Libya). J. Water Resour. Prot. 2015, 7, 369–377.
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Shi F, Zhao C, Sun D, Peng D, Han M (2012) Conjunctive use of surface and groundwater in central Asia area: a case study of the Tailan River Basin. Stoch Environ Res Risk Assess. 26:961–970.
Siemens, M.; Dynes, J.J.; Chang, W. Sodium adsorption by reusable zeolite adsorbents: Integrated adsorption cycles for salinized groundwater treatment. Environ. Technol. 2020, 1–41.
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110.
Tweed, S.O., Weaver, T.R. and Cartwright, I. (2005): Distinguishing groundwater flow paths in different fractured-rock aquifers using groundwater chemistry: Dandenong Ranges, Southeast Australia. Hydrogeology Journal; 13: 771-786.
Tuinhof, A.; Foster, S.; van Steenbergen, F.; Talbi, A.; Wishart, M. Appropriate groundwater management policy for Sub-Saharan Africa. Appropr. Groundw. Manag. Policy Sub. Saharan Africa 2011.
Vousoughi FD, Dinpashoh Y, Aalami MT, Jhajharia D (2013) Trend analysis of groundwater using non-parametric methods (Case study: Ardabil plain). Stoch Environ Res Risk Assess 27:547–559.
Wheida, E.; Verhoeven, R. An alternative solution of the water shortage problem in Libya. Water Resour. Manag. 2007,21, 961–982.
WHO. (2022). Guidelines for drinking-water quality: incorporating the first and second addenda: World Health Organization.
Zhu, C., Schwartz, W., 2011. Hydrogeochemical processes and controls on water quality and water management. Elements 7 (3), 169–174
