Histogenesis of suprarenal gland, kidney and liver in mice Mus musculus
Dina H. Sadiq1
Nursing collage /Basra University/ Iraq
HNSJ, 2024, 5(9); https://doi.org/10.53796/hnsj59/18
Published at 01/09/2024 Accepted at 20/08/2024
Citation Methods
Abstract
Objective: The postnatal development of the suprarenal glands, liver, kidney in mice was examined in this work using histological and histochemical techniques. For this reason, it is acceptable to use mice in scientific study. Materials and methods: Thirty two samples of mice’s suprarenal gland, liver, and kidney; eight at each age were used in the experiment (one day, seven day, fourteen day, twenty one day). Results: The adrenal glands were enclosed in a well-developed capsule, the cortex was divided in the glomerulosa, fasciculate, and reticularis areas, and the medulla consisted of large, pale-stained hexagonal cells arrangements in tiny anastomosing strings had together by a reticular fiber and divided through sinusoids in all ages with varying thickness of the layers. When the first day of life; liver was enclosed in a thin capsule; after 14 to 21 days, it was enclosed in a thick capsule. The parenchyma consisted of hepatocytes, which were surrounded by a central vein. The sinusoids further divided the parenchyma, with Kupffer cells protruding next to the endothelial cells that bordered the hepatic sinusoids. Depending on the age, the liver measurements changed. There was a modest amount of collagen fiber covering the kidney. Renal corpuscles are classified as cortex, midcortical, or juxtamedullary; as they age, their diameter increases. Proximal convoluted tubules are the longest; they have a brush border and are surrounded by cuboidal epithelial tissue; distal convoluted tubules are shorter. Additionally, henle loops were long at some ages but short at one-day and adult ages. The corpuscle and nephron lumens were bigger in adults. In contrast, there were less collagen fibers in the thick renal capsule and gap in other age groups.
Key Words: developmental, histochemical, mice, suprarenal gland, liver, Kidney
INTRODUCTION
The medulla, which originates from neural crest cells near the dorsal aorta, and the cortex, which develops from the intermediate mesoderm, are the two distinct embryological structures from which the adrenal glands emerge (1). By producing catecholamines and steroids, the adrenal gland controls several vital physiological processes in the adult body. Integration of extracellular and intracellular signals controls maintaining of adrenal structure and function. Adrenocorticotropic hormone (pituitary hormone), the primary hormone promoting adrenal glucocorticoid production and secretion, is essential to this control. Basic and clinical researchers will be interested to comprehend the mechanisms underlying adrenal growth and development, maintenance, and regeneration because significant progress has been made in knowing the structure of the process of development and the remarkable regenerating capacity of the adrenal gland (2). Hepatocytes within the liver lobes, which are separated by circulatory channels and eventually extend into sinusoids, are derived from the major alimentary tract. The liver is the primary organ responsible for preserving metabolic homeostasis since it carries out vital functions like metabolism, bile secretion, the process of detoxification and plasma protein synthesis (3-8). Mammals kidneys develop in three stages, each of which is distinguished by the development of a more developed pair of kidneys: the pronephros, mesonephros, and metanephros (9). Mesenchymal condensates are the first step in the creation of the mesonephric nephron. Renal vesicles and S-shaped structures quickly form from these condensates. It has been established that metanephros underwent similar developmental stages. The Wolffian duct and the collecting duct divide the mesenchymal origin of the various nephron segments (10-13). The kidneys of domestic animals are big, solid, and shaped like beans (14).Which enables it to obtain a huge amount of information when searching for any topic using search sites, whether at home or office (10) . Nursing is the sum of services given to individuals and their families to help them maintain their natural state or help them to relieve their organic and psychological pain (34).
MATERIALS AND METHODS
Ethical approval
Every processes were carried out in the accordance with animal care guidelines, University of Baghdad, Iraq, during March to May 2024.
Study animals: Thirty two samples of the suprarenal gland, liver and kidney from mice were use in a experiment during March to May 2024, eight animals at each of age; one day old as freshly born; seven day age as nursing, fourteen day age as weaning, and twenty one day age as adult mice .
Samples collection:
An overdose of injectable ketamine is used to induce anesthesia in all ages, except one day. Every animal had its liver, kidney, and adrenal (suprarenal) gland removed from ventral abdominal wall. The midline of abdomen had been cut, and the visceral organs have been eliminated to facilitate easier access to the liver, kidney, and adrenal gland. The research organ samples underwent histological examination (21).
Histological technique: dehydration by ethyl alcohol, xylene for cleaning, paraffin wax for infiltration and embedding, using microtome for sectioning (22).
Tissue staining: Hematoxylin and Eosin (H @ E); verhofes; Masson’s trichrome and mallory were the stains used (22).
Histochemical staining:
Periodic Acid Schiff (PAS) was used to treat mucopolysaccharides and glycoproteins. For thirty minutes, sections were submerged in an aqueous solution including 1% periodic acid. After being cleansed to eliminate any remaining acid, the slices were exposed to twenty minutes of Schiff’s reagent and one minute of potassium metabisulfite 0.55%. Alcian blue (pH 2.5): Follow these steps to acidic mucopolysaccharides: Use xylene to deparaffinize the material. After thirty minutes with alcian blue, five minutes with tap water, and ten minutes with nuclear fast red, rehydrate in graded ethanol. Take a minute to use the water. achieving dehydration by using an ethanol gradient. The resulting Alcian Blue Addition Periodic Acid Schiff (AB-PAS) approach, which entailed deparaffinizing and rehydrating with distilled water, was then used to compare both neutral and acidic mucopolysaccharides. Microwave; pH 2.5; alcian blue. For standing, allow two to five minutes, and for using maximum force, allow 45 seconds. After washing with the faucet for about five minutes, rinse with distilled water. For five minutes, work with 0.5% periodic acid. Use de-ionized water for washing. After 45 seconds of intense activity, take two to five minutes to recover using Schiff’s Reagent and the microwave. Rinse with distilled water after five minutes of washing under running water(22).
STATISTICAL ANALYSIS:
The one-way analysis of the variance (ANOVA) test used to examined study at the 5% significant level. Data handled and examined using social science statistical software (23).
RESULTS AND DISCUSION
The cortex and the medulla make up the adrenal gland. The mesenchymal cells that make up the adrenal capsule encircle the cortex. The most outermost zona glomerulosa, the middle zona fasciculate, and the most inner zona reticularis are the three distinct cortical zones that lie beneath the capsule (Fig. 1-4), which, despite their disparate roles and developmental genesis, are essential to an organism’s lifespan because they regulate endocrine system (24). (1) Describe how the adrenal glands descend after developing in the peritoneum. Through a number of biological metabolic processes, the adrenal cortex converts cholesterol into steroid hormones., sympathetic nervous system includes the medulla, which is responsible for producing norepinephrine and adrenaline (24). While the medulla comes from the neuroectoderm, cortex develops from mesodermal tissue (1). The adrenal gland’s developmental program starts in the embryo and continues through the fetus and postnatal animals (2). During the postnatal period, the adrenal gland continues to go through major remodeling. The zona fasciculata immediately after birth experiences apoptosis in its cells, which causes the surviving zona fasciculata to involute. On the other hand, the adult zona fasciculata and zona glomerulosa mature in response to the growth hormones angiotensin II and ACTH. Subsequently, the adrenal zona reticularis, which is distinguished by enhanced adrenal androgen synthesis and proliferation, forms between zona fasciculata and medulla. When puberty begins in rodents, the remaining pieces of the zona fasciculata also known as the X-Zone in mice finally retreat (1,25).
Age-related variations in adrenal layer thickness include smaller values on first day of life compared to later ages (Table 1). The adrenal cortex is involved in the regulation of several physiological processes, such as energy metabolism, salt metabolism, and stress response, through the release of steroid hormones. Aldosterone secretion is a mineralocorticoid that must be carefully controlled and adapted to the body’s requirements. In outermost layer of adrenal cortex, angiotensin II or a rise in plasma K+ concentrations that trigger the renin-angiotensin-aldosterone system are the two main stimulants of aldosterone synthesis and secretion. Aldosterone is essential for regulating blood pressure and the composition of plasma electrolytes because it causes the kidney to reabsorb Na+ and excrete K+ (1).
Renin transforms angiotensinogen into angiotensin I, which is then transformed into angiotensin II in the lung by an angiotensin converting enzyme. In target tissues such as the colon, salivary gland, renal distal convoluted tubule, and collecting ducts, aldosterone binds to the nuclear receptor mineralocorticoid receptor, causing an increase in sodium reabsorption and an increase in potassium excretion by principal cells and hydrogen ion excretion by intercalated collecting duct cells. The zona fasciculata cells generate glucocorticoids. Under normal circumstances, the adrenal cortex produces the majority of the glucocorticoids, cortisol, which has the ability to mobilize lipids, proteins, and carbohydrates. Corticosterone is the primary glucocorticoid generated and used in mice and rats (2,25)
Mice’s kidneys expand continuously from birth and show further morphogenesis after seven days. The glomerular condensation in the cortical and subcortical zones of the renal cortex varied, with the periphery growing more quickly than the mid-cortical and subcortical zones. Renal corpuscles were more numerous in the cortical and subcortical regions of the kidney than in the juxtamedullary zones. Bowman’s capsules and a noticeable parietal squamous layer were visible in the corpuscles. In both the proximal and distal convoluted tubules, more development was seen. At this age, the medulla featured a collecting duct surrounded by cuboidal epithelium, clear intercellular membranes, slightly stained cytoplasm, and nuclei in the center (Fig. 5,6). This is consistent with (26), which states that kidneys grow in the pelvis and rise (3). This was therefore linked to changes in tubule activity about water quality. Proximal tubules were additionally loaded with delicate secretions to enhance the volume of fluid consumed, recover more components from the lumen into the circulation, and raise the surface area of tubule lumen. Additionally, practically all vitamins, ions, minerals, and plasma proteins are absorbed by the proximal tubule cells (27).
The kidney has improved renal structure establishment and morphogenesis at day fourteen. There were clear Bowman’s capsules and the parietal squamous layer visible. At the conclusion of this time, every nephron component has grown into its own distinct histology. The brush border of the proximal convoluted tubules epithelium is well developed, and the Henle loops are clearly visible. The kidney lobe, tubules, collecting ducts, and medullary rays were observed ascending toward the subcortical and cortical zones, forming the renal pyramids in the medulla. The renal papilla, which forms as the circular apex of the medullary pyramid descends from the base, is surrounded by low cuboidal epithelium in collecting tubules (Fig. 7-11). This is analogous to (28).
The kidney of twenty one-day-old mice was found to have a fully developed renal parenchyma structure. The interstitial tissue capsule contains collagen fibers, which make up a large portion of the stroma. It was discovered that the appropriate morphology of renal corpuscles originates from the parietal and visceral layers of Bowman’s capsule, which are separated from one another by the characteristic Bowman’s gap around the glomeruli. The distinctive eosinophilic cytoplasm of the lining cell of the proximal tubules indicates differentiation. Renal loop elongation, ducts, and collecting tubules were located in the denser medulla. In adults, the kidney was enclosed in a capsule of collagenous fibrous tissue (Fig. 5-11). The adults kidney, which is in charge of cleaning blood in circulation, had a considerable blood flow and was thicker than in other ages with the significant difference (P>0.05) (Table 1). These alerts bore similarities to (29).
In the present study, a connective tissue capsule was used to control kidney size and shape according to the age of the animal. The glomerulus was positioned subcapsularly at various embryonic stages and seems to have been invaginated in Bowman’s capsule. The diameter of the Bowman’s gap varied (Fig. 10). (4,30) provided verification of these. The current work reports that the kidney of an adult mouse at twenty-one days of age possesses the usual morphology of an adult nephron. There were visible podocytes around the glomerular, and the juxtaglomerular apparatus, which is located at the vascular pole of the renal corpuscles, is made up of distal tubules with a big lumen (Fig. 10,11). This is consistent with (31)’s findings. Because it creates an osmotic gradient between the cortex and medulla, which is what propels the extraction of water from the urine, this structure is critical to the operation of the metanephric kidney. The renin enzyme is released throughout the circulation as a result of chemical signals transmitted by the macula dense cells. These cells are highly sensitive to the fluid volume in the tube as well as the ion concentration, acting as vasoconstrictor cells. Mesangial cells carry out phagocytic functions during these processes (32).
Hepatocytes comprised up the parenchyma surrounding the liver, which was covered in a simple squamous epithelium. Kupffer cells protruded adjacent to the endothelial cells that lined the sinusoids that separated the principal vein encircling the parenchyma (Fig. 12,13). Age-related variations in the thickness of the hepatic artery, central vein, and hepatic duct are observed, with adulthood exhibiting a larger variance than other ages (Table 1). The liver showed faster morphogenesis and the structural formation of hepatic cells at thirty days of life. There are few connective tissue septa between the lobules that make up the liver. It is unexpected where the core veins are located within the lobules. Hepatocytes were polyhedral in form and contained spherical nuclei organized in irregular cords. The portal triads, located in the angles of the hepatic lobules, are made up of the hepatic artery, bile duct, and branch of the portal vein (Fig. 14-16). According to research (8,33), the adult liver has a wider portal triad than livers of other ages (Table 2). Elevated metabolic activity and hepatocyte activities, which are associated with age-related differences in surface area and size and promote the maintenance of body homeostasis, could be the cause of this. According to a number of studies on the liver, environment, and embryonic development all affect the shape of the liver, as seen by the way animals adapt to their environments (4). As evidenced by their absorption of foreign objects and poisonous compounds which enter through the portal artery, kupffer cells have a defensive physiological role (33).
According to histochemical studies, PAS, AB, and PAS-AB were well-received by the cells of the adrenal cortex, adrenal medulla, kidney, and liver. The glomerulus’s basement membranes and the proximal tubules’ brush border were both shown to be well-developed by PAS stain. Where the renal tubules encircled the renal corpuscle in the renal cortex, the mature organization of the renal components was seen. The glomerular capillaries were observed to be surrounded by the parietal and visceral layers of Bowman’s capsule. The spaces between renal corpuscles were filled with renal tubules of varying diameters. As a part of the juxtaglomerular apparatus, distal tubules with a large lumen were discovered close to the vascular pole of the renal corpuscle. The hepatic cells and the kidney’s brush border responded well to PAS, AB, and PAS-AB. The renal tubules and glomerulus basement membranes were stained with PAS (Fig. 1, 2, 6, 9, 10), which is consistent with previous studies (2, 8, 32).
Table (1): Measurements of adrenal gland and kidney in mice, μm (X– ± S.E)
| distal tubules | Proximal tubules | glomerulus | Capsule of kidney | medulla | Cortex | Capsule of adrenal gland | Species
Part |
| 32.2±0.01 B | 74.3±0.02 B | 122.4±0.3b | 37.7±2.3B | 102.3±0.04 B | 901.1±1.2 B | 24.6±0.1 B | 1 day |
| 39.4±0.02 B | 83.2±0.01 B | 127.5±0.2b | 40.5±1.5B | 112.5±0.06 B | 915.2±1.6 B | 26.3±0.4 B | 7 day |
| 42.2±0.07 B | 88.5±0.04 B | 132.6±0.1b | 43.2±0.5B | 122.3±0.07 B | 924.4±1.4 B | 30.1±0.2 B | 14 day |
| 53.1±0.02 B | 97.2±0.03 B | 139.7±0.2b | 47.6±2.3B | 127.2±0.04 B | 932.6±1.5 B | 33.5±0.6 B | 21 day |
Table (1): Measurements of liver at different ages, μm (X– ± S.E)
| Hepatic duct | Central vein | Capsule | Measure
Age |
| 239.1±0.4 B | 531.4±2.4 B | 67.1±0.3 B | 1 day |
| 255.8±0.6 B | 550.5±1.1 B | 73.4±1.5 B | 7 day |
| 252.7±0.5 B | 582.3±2.3 B | 103.6±0.1 B | 14 day |
| 260.2±0.3 B | 625.1±2.7 B | 132.4±1.2B | 21 day |
The values columns with capital letters in same column denote for significant difference (P>0.05), while column values with small letters denote to nonsignificant differences (p<0.05).
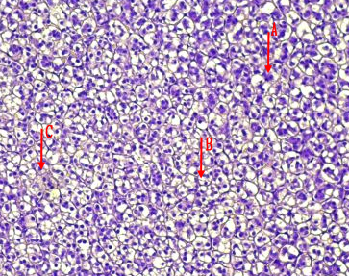
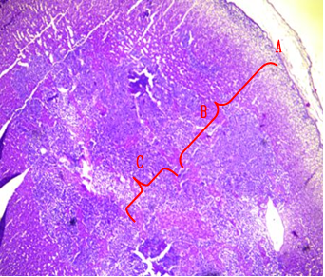
Fig.(1): Light micrograph of adrenal gland, at 1 day age; capsule (A), cortex (B), medulla (C), Masson 100X.
Fig.(2): Light micrograph of adrenal gland, at 7 day age; glomerularis cells (A), fascularis cells (B), connective tissue (C), PAS 200X.
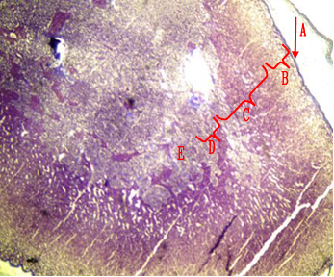
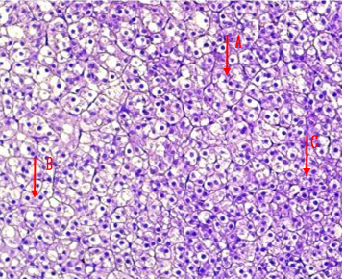
Fig.(4): Light micrograph of adrenal gland, at 21 day age; glomerularis cells (A), fascularis cells (B), connective tissue (C), AB 200X.
Fig.(3): Light micrograph of adrenal gland, at 14 day; capsule (A), glomerularis (B), fascularis (C), reticularis (D), medulla (E),, Mallory 100X.
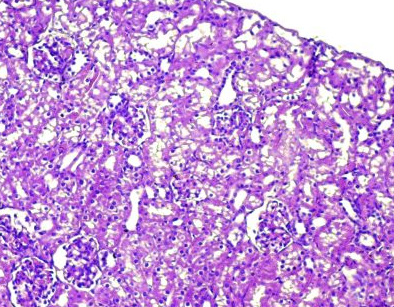
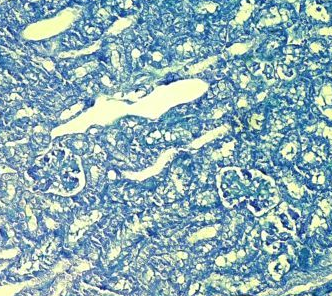
Fig.(6): Light micrograph of kidney, at 7 day age; glomerulus (A), Bowman capsule (B), renal space (C), proximal tubule (D), distal tubule (E), AB 200X.
Fig.(5): Light micrograph of kidney, at 1 day age; capsule (A), glomerulus (B), Bowman capsule (C), renal space (D), proximal tubule (E), distal tubule (F), PAS-AB 200X.
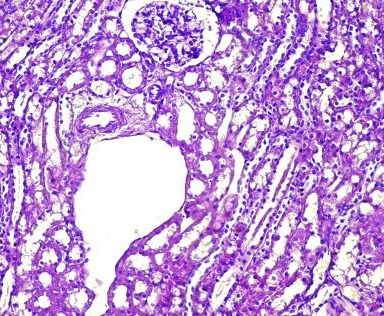
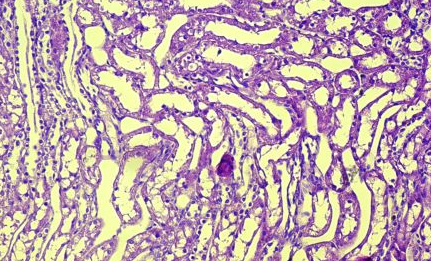
Fig.(7): Light micrograph of kidney, at 14 day age; glomerulus (A), renal space (B), Bowman capsule (C), juxtaglomerulr apparatus (D), blood vessels (E), PAS-AB 200X.
Fig.(8): Light micrograph of kidney, at 21 day age; loob of Henle (A), proximal tubule (B), distal tubule (C), AB 200X.
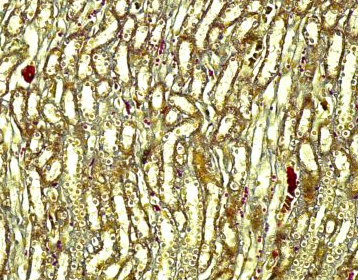
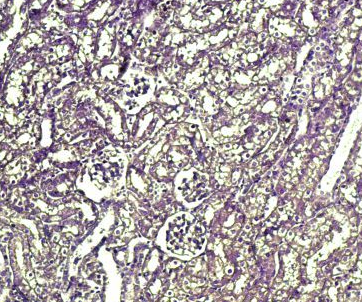
Fig.(10): Light micrograph of kidney, at 14 day age; proximal tubule (A), distal tubule (B), Henle loop (C), Verhofes 200X.
Fig.(9): Light micrograph of kidney, at 7 day age; glomerulus (A), renal space (B), proximal tubule (C), distal tubule (D), mallory 200X.
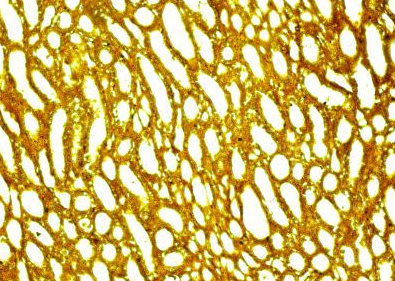
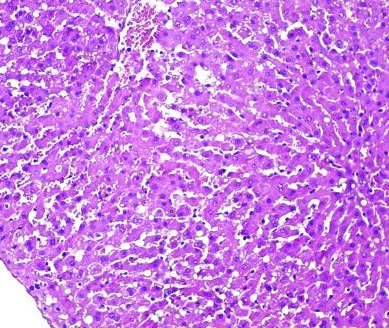
Fig.(11): Light micrograph of kidney, at 21 day age; proximal tubule (A), distal tubule (B), collecting duct (C), Verhofes 200X.
Fig.(12): Light micrograph of liver, at 1 day age; capsule (A), hepatic cells (B), sinusoids (C), central vein (D), H@E 200X.
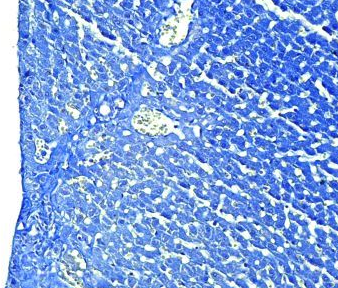
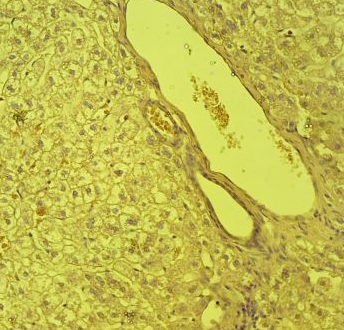
Fig.(14): Light micrograph of liver, at 14 day age; capsule (A), central vein (B), endothelium (C), hepatic cells (D), sinusoids (E), AB,400X.
Fig.(13): Light micrograph of liver, at 7 day age; central vein (A), endothelium (B), hepatic duct (C), hepatic artery (D), hepatic cells (E), sinusoids (F), Verhofes,400X.
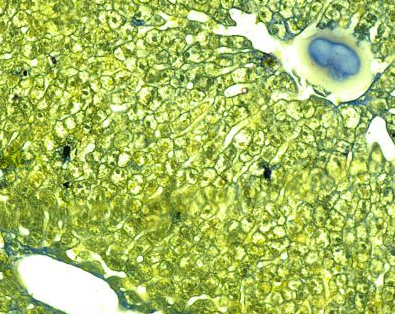
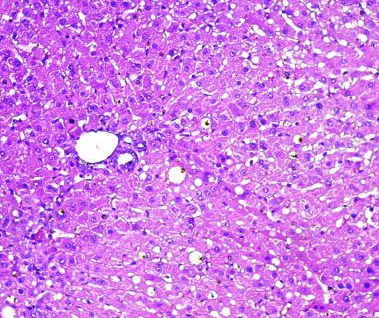
Fig.(16): Light micrograph of liver, at 21 day age; central vein (A), hepatic duct (B), hepatic cells (C), sinusoids (D), Masson,400X.
Fig.(15): Light micrograph of liver, at 21 day age; central vein (A), endothelium (B), hepatic cells (C), sinusoids (D), Verhofes,400X.
CONCLUSION: The architecture of the adrenal gland, liver, and kidneys change as animals get older. Twenty one day after delivery, these organs underwent postnatal developmental modifications before growing into the typical adult nephron..
Funding
The author provides self-supporting financing for this research, rather than receiving outside funding.
Availability of data and materials
Available data from the current study region upon reasonable request.
Competing interest
There are no conflict of interest between the authors.
Ethical consideration
The ethical guidelines for study conduct have been adhered to by the University of Baghdad, Iraq.
Author contributions
All authors contribute equally.
Acknowledgements
We would especially want to express our gratitude to the Histology Department at the University of Baghdad’s Veterinary Medicine College in Iraq for their diligent efforts and commitment to our initiative.
REFERENCES
- Xing Y, Lerario AM, Rainey W, Hammer GD. (2015): Development of adrenal cortex zonation. Endocrinol Metab Clin North Am.; 44(2):243-74. doi: 10.1016/j.ecl.2015. 02.001. PMID: 26038200; PMCID: PMC4486052.
- Jason Karpac, Dirk Ostwald, Stephanie Bui, Peggy Hunnewell, Malini Shankar, Ute Hochgeschwender,(2005): Development, Maintenance, and Function of the Adrenal Gland in Early Postnatal Proopiomelanocortin-Null Mutant Mice, Endocrinology, 146, (6): 2555–2562, https://doi.org/10.1210/en.2004-1290.
- Elsheikh, E.M. (2023): Histogenesis of the rabbit liver (pars hepatica) with particular reference to the portal area, Iraqi Journal of Veterinary Sciences, 37 (1): 177-182.
- Yousif N H, Hadi H D, Jihad H M. Histological study of liver in guinea pig Cavia porcellus ( Linnaeus , 1758) in Iraq.Revis Bionatura 2023;8 (3) 80. http://dx.doi.org/10.21931/RB/2023.08.03.80.
- Yousif, N. H. Histological study of liver for squirrel (Sciurus anomalus)(Güldenstädt, 1785) in Iraq. GSC Biological and Pharmaceutical Sciences, 2022, 20(1), 091-094.
- AL-Aamery, Rana Alaa, et al. (2020). Morphological description and comparative histological study of the liver in two iraqi mammals: weasel (herpestes javanicus) and eastern gray squirrel (sciurus carolinensis). Biochem. Cell. Arch. 2020 , 20(1): 167-170
- Dyce KM, Sack WO, and Wensing CJG (2010). Textbook of veterinary anatomy.4rd ed. W. B. Saunders company. Philadelphia, Pp: 554 – 694.
- Al-Hamdany, M.Z. (2019): Comparative anatomical, histological, and histochemical study of liver in human and domestic rabbit, Iraqi J. of Vet. Sciences, 33 (2): 437-446.
- Sainio, K., (2003). Development of the mesonephric kidney. In: Vize, C., Woolf, A. S, and Bard, J. B. L., editors. The kidney. From normal development to congenital disease. London: Academic Press pp. 75–86.
- Suhett, W.G.; Gerez, J.; M.S. Hohmann, L. Staurengo-Ferrari, W.A. Verri, F.H.O. Pinho, L.D. de Barros, S.T. Cardim, K.M.C. Flaiban, Ana Paula F.R.L. (2023). Bracarense, Exploring porcine kidney explants as a model for the study of nephrotoxins and the therapeutic potential of phytic acid, Environmental Toxicology and Pharmacology,Volume 102,2023,104241,https://doi.org/10.1016/j.etap.104241.
- Dutta P, Hakimi S, Layton AT. (2024). How the kidney regulates magnesium: a modelling study. R Soc Open Sci.; 11(3):231484. doi: 10.1098/rsos.231484.
- Kaewmong P, Jongjit P, Boonkasemsanti AK, Kongtueng P, Matchimakul P, Tangphokhanon W, Pirintr J, Piboon P, Umsumarng S, Nganvongpanit K, and Pongkan W (2023). Histological study of seventeen organs from dugong (Dugong dugon) PeerJ 11:e15859 https://doi.org/10.7717/peerj.15859.
- Baragooth AF, Ghazi HA, and Abdzaid K (2014). Histological study to the nephrons of the kidney in dogs (Canis familiaris) in middle of Iraq. Kufa Jurnal for Veterinary Medical Science, 5(1):98-103.
- Kalita A and Kalita PC (2014). Urinary system of mizo local pig (zovawk): A gross morphological and morphological study. Euro. J. Bio. Parm. Sci. 1(3): 458-464.
- Madrazo-Ibarra A and Vaitla A (2020). Histology, Nephron. National Library of Medicine, National Institutes of Health pp:2-3.
- Marc-André DA, Bédard A, and Marilyn ED (2011). Clinical significance of renal pelvic dilatation on ultrasound in dogs and cats. Ultrasound. 2011;52(1):88–94.
- Marco A, Sampaio B, Beatriz P, Sampaio D, Henry R, Favorito M, and Francisco S (2009). The dog kidney as expermental model in endourology: anatomic contribution. J. endourology. 23(6): 989-993.
- Marques‐Sampaio BP, Pereira‐Sampaio MA, Henry RW, Favorito LA, and Sampaio FJ (2007). Dog kidney: Anatomical relationships between intrarenal arteries and kidney collecting system. Anat Rec.;290(8):1017-22. DOI: 10.1002/ar.20567.
- Maurya H, Kumar T, and Kumar S (2018). Anatomical and physiological similarities of kidney in different experimental animals used for basic studies. J Clin Exp Nephrol.;3(09). DOI: 10.21767/2472-5056.100060.
- Mukoyama M and Nakao K (2005). Hormones of the kidney. In: Melmed S, Conn PM, editors. Endocrinology. USA: Springer: 353-65 .
- Mahmood MB. (2022). A comparison between ketamine-xylazine and ketamine-midazolam or all of them to induce balance anesthesia in rabbits. Iraqi J Vet Sci.; 36(2): 499-506. DOI:10.33899/ijvs.2021.130618.1852.
- Suvarna SK, Layton C, and Bancroft J D (2018). Bancroft,s theory and practice of histological techniques, 8th ed. Churchill Livingstone Elsevier Philadelphia, Pp: 176 -725.
- Al-Rawi KM and Kalaf-Allah IS (1980). Design and Analysis Agriculture Experiments. Dar-Al Kutub-Mosul, Iraq, Pp: 65, 95-107.
- Plain, A., Knödl, L., Tegtmeier, I. et al. The ex vivo perfused mouse adrenal gland—a new model to study aldosterone secretion. Pflugers Arch – Eur J Physiol (2024). https://doi.org/10.1007/s00424-024-02950-z.
- Abass,T. A. (2017). Anatomical and histological study of adrenal gland in new natal and adult guinea pig (Cavia porcellus). Kufa Journal for Veterinary Medical Sciences, 8(1):181-192.
- Ekele, I; Uchenna, N and Ibe C.S. (2014). The Kidney and adrenal gland of the African Palm Squirrel ( Epixerus ebii): A Microanatomical Observation.Rev. Fac. Cs. Vets. 55(2):60-67.
- Rossi, G.; Liu, K.-F.; Kershaw, H.; Riddell, D.; Hyndman, T.H.; Monks, D.; Musk, G.C. (2023). Biological Variation in Biochemistry Analytes in Laboratory Guinea Pigs (Cavia porcellus). Vet. Sci., 10, 621. https://doi.org/10.3390/vetsci10100621.
- Nawata MC and Pannabecker T L (2018). Mammalian urine concentration: a review of renal medullary architecture and membrane transporters. J. Comp. Physiol. B. ;188(6):899–918.
- Seema S, Rakesh M, Sanjeev J, Geeta BR, and Vikas K (2017). Histological Study on capsule of the kidney in large white Yorkshire Pig (Susscorfa); Indian Journal of Veterinary Anatomy 28(2):29-30.
- Yang IS, Jang I, and Yang JO (2023) CanISO: a database of genomic and transcriptomic variations in domestic dog (Canis lupus familiaris). BMC Genomics 24, 613. https: //doi. org/10.1186/s12864-023-09655-0.
- Ebeid TA, Aljabeili HS, Al-Homidan IH, Volek Z, Barakat H. Ramifications of Heat Stress on Rabbit Production and Role of Nutraceuticals in Alleviating Its Negative Impacts: An Updated Review. Antioxidants. 2023; 12(7):1407. https://doi.org/10.3390/antiox12071407.
- Kaewmong P, Jongjit P, Boonkasemsanti AK, Kongtueng P, Matchimakul P, Tangphokhanon W, Pirintr J, Piboon P, Umsumarng S, Nganvongpanit K, and Pongkan W (2023). Histological study of seventeen organs from dugong (Dugong dugon) PeerJ 11:e15859 https://doi.org/10.7717/peerj.15859.
- Zhou Z, Xu MJ, Gao B. (2016). Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol;13:301-315.
- Luaay abdulwahid shihab, ISRAA HUSSIN ABD, Zeinab Faisal Abd, Zahia Abdel-Hussein Masatar, w (2018), Evaluation of the nurses’ knowledge about the internet, Journal of Network Computing and Applications 3: 1-7 Clausius Scientific Press, Canada.
