Detection of fungi that causes foot ulcer for diabetes patients
Ehab Y. Jabber ¹,Ghufran f.aljuburi 2, Salim S. Jaafar3 , Safa M. Dyaa4
University of Babylon, DNA research center, Babylon , Iraq
2 Dept. Pathological Analysis, Faculty of science, Kufa University, Najaf, Iraq
3 University of Babylon, DNA research center, Babylon , Iraq
4 University of Babylon, DNA research center, Babylon , Iraq
*Corresponding author’s E-mail:pre260.ehab.yehaa@uobabylon.edu.iq
HNSJ, 2024, 5(9); https://doi.org/10.53796/hnsj59/23
Published at 01/09/2024 Accepted at 20/08/2024
Citation Methods
Abstract
This research was carried out for the purpose of isolation and identification of fungi. Isolation of diabetic patients with foot ulcers using various detection methods, including direct examination and laboratory culture. 30 foot ulcers were collected from diabetic patients at AL-Hashimiya General Hospital in Babylon during the period from January 2024 to March 2024. The percentage of women and men was as follows: women (17) 15 (62.5%) infected, 2 (33.33%) uninfected, and men (13) 9 (37.5%) infected, 4 (66,%) 67) uninfected infected between the ages of 15-70. This study found that the rate of fungal infection was higher in women than in men and was more common in patients between the ages of 20 and 68. When culturing samples of Sabouroud dextrose agar (SDA) and potato dextrose agar secondary media (PDA), the results showed the presence of 24 isolates among them: – 10 (41.67%) Candida spp., 8 (33.33%) Alternaria spp. 6 (25%) of Rhodatorula spp.
Based on this result, Candida spp. is the most frequently an isolated species from other isolates such as Alternaria spp. Rhodatorula spp.
Key Words: Fungal infection , Diabetic food ulcer, Alternaria alternate , Candida spp.
1-Introduction
Foot infections are common and usually not very serious. Fungal foot infections can lead to potentially fatal and limb-threatening consequences, which is more common in those with diabetes (1). Fungi are found in practically every environment and may thrive in a variety of temperatures and pH ranges (2). Hyperglycemia, or high blood sugar, is the hallmark of people with diabetes mellitus (DM), a metabolic disease caused by a defect in the insulin-secreting systems, insulin resistance, or both. Chronic hyperglycemia in people with diabetes results in Chronic damage, dysfunction and failure of many organs, including the heart, blood vessels, nerve cells, kidneys and eyes. (3).
Uncontrolled blood sugar levels increase the risk of foot infections in diabetics by causing peripheral neuropathy and compromising blood circulation, particularly in the lower extremities (4). In addition, because they are unable to feel cuts and irritations on their feet, patients are unaware of the infection (5). DFUs are linked to fungus infections, and this association is more likely due to the polymicrobial character of DFUs (6). Only a small number of research have focused on the frequency of fungal colonies in DFUs. Fungal infections are found in nearly 25% of DFUs, however because DFU clinics employ conventional microbiology laboratory protocols, these infections are frequently missed or misdiagnosed (7).
Aims of study:
1. Determining the primary reason behind diabetic foot ulcers.
2. Determine which fungus cause ulcers.
2. Materials and Procedures
Collection of specimens
Samples were obtained from diabetic foot wounds of diabetic patients attending Al-Hashimiya General Hospital. In the city of Al-Hilla, Babil Province, Iraq, from January to March 2024. The ages of the patients in this study ranged from 15 to 70 years, and they were of different genders.
The area around the wound was thoroughly cleaned with 10% povidone-iodine and then sterile normal saline before a culture was taken. Using a swab and transport media, cultures were obtained from the ulcers’ depths. In less than an hour, a tissue sample was gathered, sealed, tagged, and delivered to the microbiology lab.
Laboratory diagnosis
Direct examination
Skin fragments from ulcer specimens were directly examined by putting the sample on a glass slide, adding drops of potassium hydroxide (KOH) 10%, and then covering the slide with a cover. The slide was then either left in the lab temperature for five minutes or gently warmed with a burner’s flame while looking for fungal hyphae (8).
Indirect examination (culture of specimen)
Samples taken from the deep areas of the diabetic foot were grown at a pH of 6.5 on the culture medium (Sabauraud dextrose agar) and incubated for seven days at a temperature of 28°C and monitored and examined daily.
Identification
isolates were identified following the following criteria
1- Colony color and consistency
2- Microscopic features (microconidia and macroconidia, their size, arrangement, shape, conidia formation).
3- Inversion of color (color change with age)
Macroscopically identification of fungi
Check the morphological characteristics of the colonies, such as size,color,and morphology of the colonies and production of hyphae to identify two specific of yeast on Sabouraud dextrose agar incubated at 37 C for 48 hours and one organisms were mold on Sabouraud dextrose agar incubated at 37 C for 7 days.
Microscopically identification of fungi
Lacto phenol cotton blue use to stain molds and yeast finally examined under 40 x.
4-Results&Discussion
Patients
In our under study, 24 infected samples were collected from 30 samples of diabetic patients suffering from foot inflammation at Al-Hashimiya General Hospital in Babil Governorate. It was collected from 15 women (62.5%) and 9 men (37.5%), and their ages ranged from (15-70) years. As shown in Table (1). Many reports have indicated that diabetes mellitus is susceptible to fungal diseases (10). Diabetes is a predisposing factor for fungal infections, especially those caused by Candida albicans. (11)
Table (1) Distribution of fungal positive cultures
| Diabetes patients state | No. Positive | |
| Infected female | Infected male | 24 (80%) |
| 15 (62.5%) | 9 (37.5%) | |
Isolation
Out of 30 specimens of all study subjected only 24 % yielded growth of fungi isolates and considered as positive specimens which were used in the phenotypic diagnosis as shown in table (2) ). In briefly they were included :- 10 (41.67%) belong to Candida spp. , As seen in figure (1), 33.33% of the isolates are from Alternaria spp., and 6 (25%) are from Rhodotorula spp. , C. albicans is the most common cause of invasive fungal infections (12). the results of this study are consistent with (13, 14), in comparison with the research by (15). A widespread environmental yeast that can be found in soil, air, and other common areas is called Rhodotorula. It is a member of the phylum Basidiomycota and has been observed to colonize humans, other mammals, and plants. The most frequent fungi isolated from diabetic foot ulcers appear to be Candida species, according to the findings of this study and earlier investigations. Rhodotorula is a genus that contains eight species, three of which are known to be harmful to humans: R. minuta, R. glutinis, and R. mucilaginosa. (16).
The majority of patients with diabetic foot ulcers in our study were male, which is consistent with the findings. (17, 18) In our work, the overall percentage of fungal positive cultures was 80%; comparable results were observed in (19). Diabetic patients are more vulnerable to infections because of the hyperglycemic environment and weakened immunity. Rhodotorula spp. is a common yeast that causes infections in people with reduced immune systems (20).
In our investigation, we verified a case of cutaneous Alternariosis. While Alternaria frequently acts as an opportunistic infection in immunocompromised hosts, it’s equally critical to acknowledge that it may pose a threat to immunocompetent hosts. Patients with diabetes may get fungal infections that allow bacterial pathogens to enter the bloodstream. (21).
Table (2) fungi isolated in Diabetic foot ulcer
| Fungi isolated | Number (%) |
| C.albicans | 10(41.67%) |
| Alternaria spp. | 8(33.33%) |
| Rhodotorula spp | 6(25%) |
| Total | 24(100%) |
Figure (1): The total percentages of the fungi isolates
Identification of Candida species isolates: – including
Morphological and cultural characteristics
samples containing Candida colonies were grown on SDA agar Depending on the species, they might be beige to pale yellow in color, develop quickly, mature in two days, and have a smooth, shiny or dry, wrinkled feel. These results were agreed with (22) as shown in Figure (2), the most abundant genus of yeasts is Candida albicans. Together with the molds Alternaria alternata in addition to Rhodotorula spp.as shown in Figure (3), Rhodotorula generates unicellular blast conidia devoid of pseudo hyphae and hyphae, as well as pink to red colonies (23).
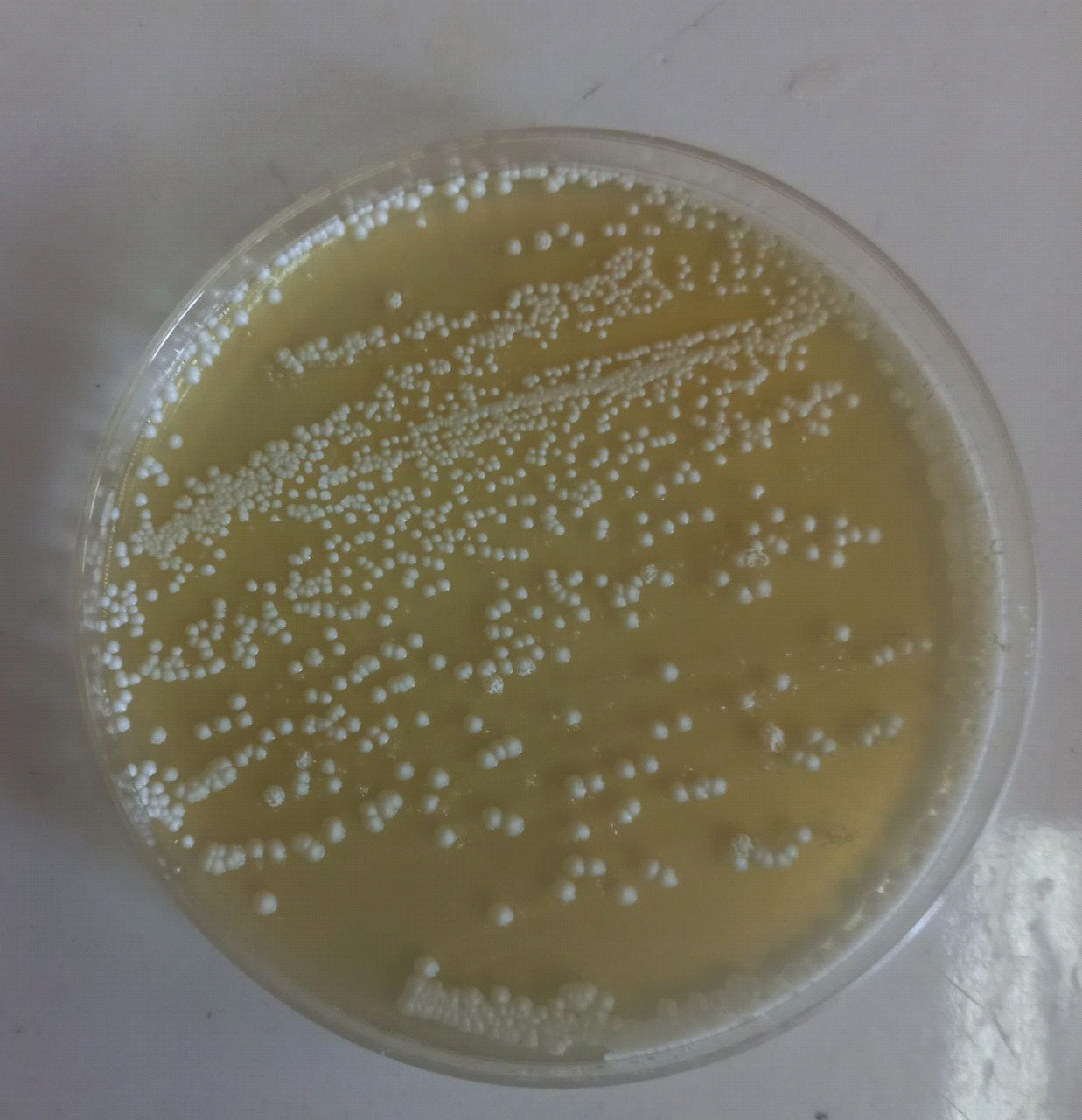
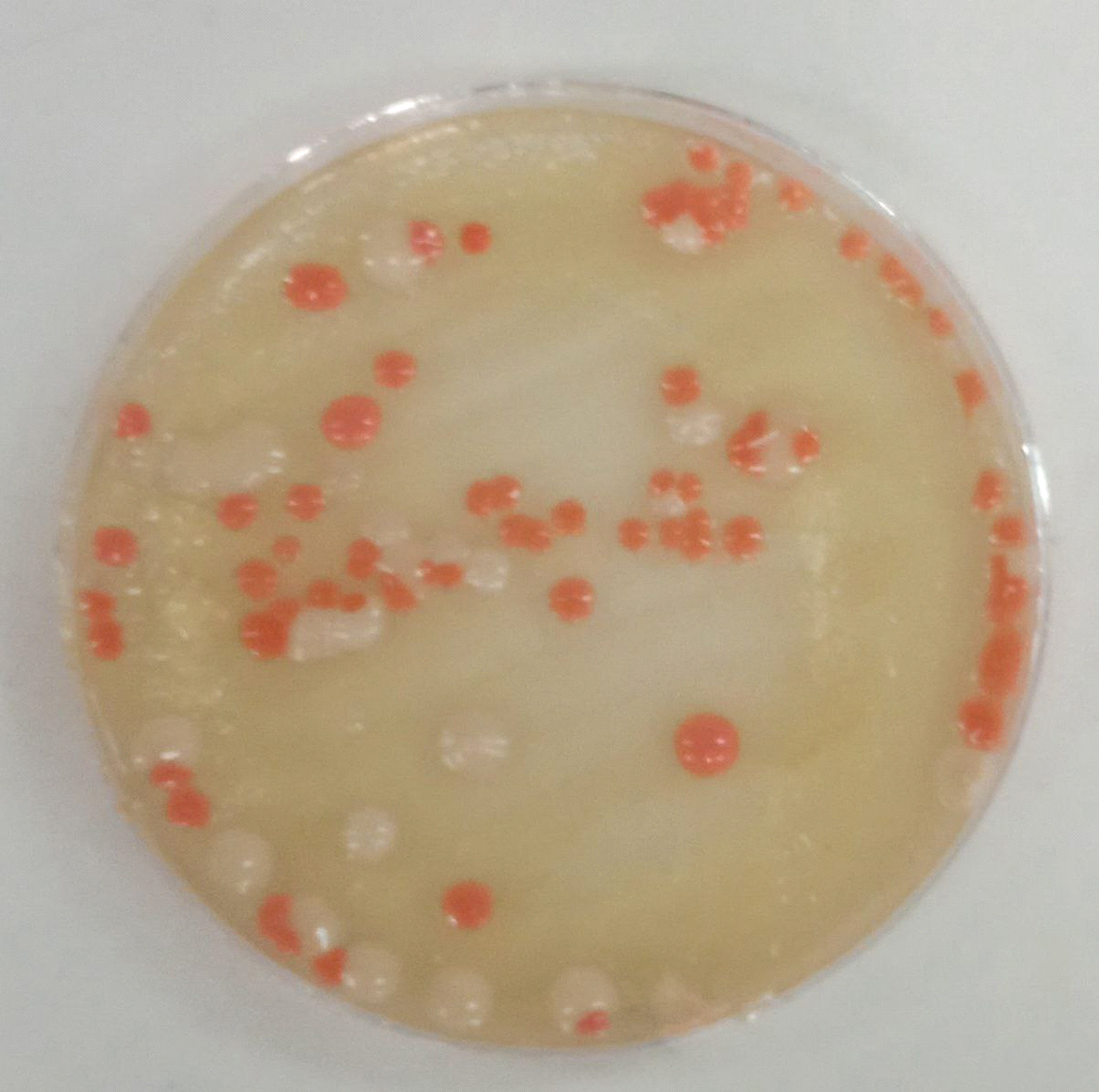
Figure (2) Yeasts grown on Sabouroud dextrose agar (SDA) at 37°C after (48) hoursof incubation A-Candida spp. B- Rhodotorula spp.
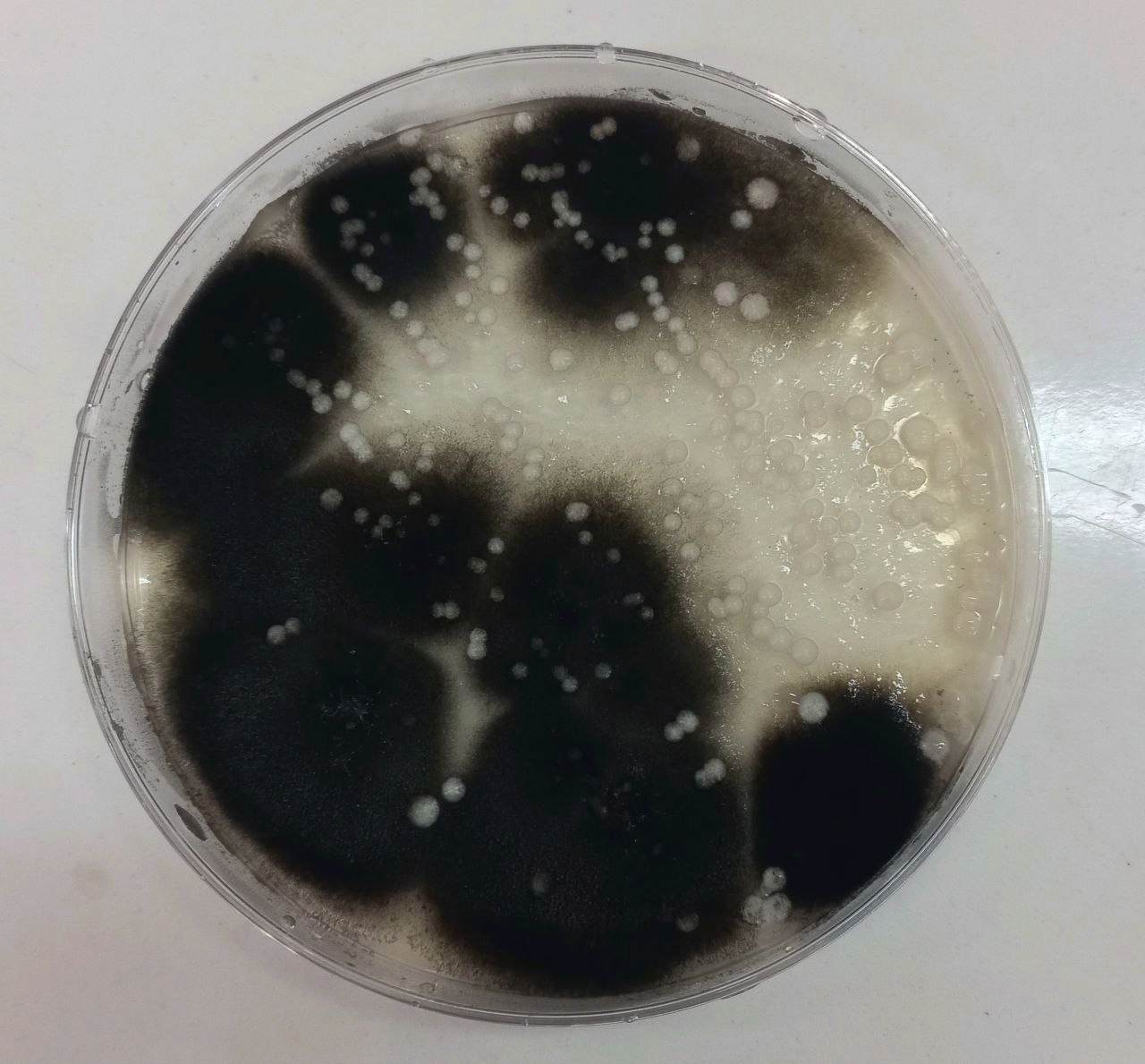
Figure (3) shows Alternaria alternata growing on Sabouraud dextrose agar at 25°C after 7 days of incubation.
Microscopic Features
All isolates were examined using an optical microscope, and true and false hyphae, unicellular blastoconidia, and chlamydoconidia of Candida were identified. (24). It has been proven that all most species produce long, branched or curved hyphae, and it has been observed that some Candida strains produce true hyphae and chlamydial spores. (25). Alternaria spp. develops fast at 25 degrees Celsius, with colonies measuring 3-9 cm in diameter after seven days. The colonies are flat, fuzzy, or hairy, and gradually cover themselves with light gray, short aerial hyphae. The surface starts off-white and darkens to green-black or olive-brown, with a lighter edge. The back is often brown to black owing to pigment production. (26). Red yeast colonies are described as velvety, smooth, moist, and even sticky. Red yeast is a low-nutrient strain that grows well on most medium and has a fast growth rate. Under the microscope, they seem as round or oval bud cells with a few pseudo hyphae. Weak capsules are occasionally generated. Red yeast generates urease but does not digest carbohydrates. They differ from Cryptococcus species by their inability to absorb inositol, and from Candida species by their failure to create pseudo hyphae and the production of colored colonies..(27) as shown in Figure (4).
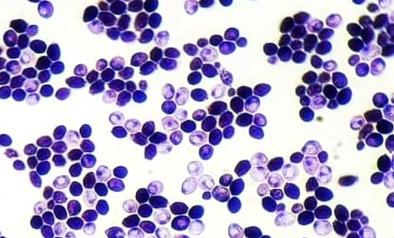
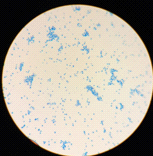
- (B)
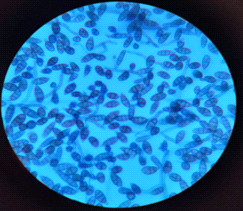
(C)
Figure (4) Characteristics of microscopic yeasts under pressure (100x) A-Candida spp. B- Rhodotorula spp. C- Alternaria spp. stained with lactophenol cotton blue
These organisms are widespread in nature (fungal species) and are opportunistic pathogens, and regardless of the age and/or sex of the patient, infection depends on the virulence of the strain and the condition of the host (28). Because smoking has a harmful effect on the respiratory and immune systems, diabetics and smokers are more susceptible to fungal infections than non-smokers.29).
Conclusion
The risk of fungal foot infections in diabetic patients, if neglected and not treated, leads to serious complications and consequences, such as foot ulceration, which leads to amputation .
Author contributions
Conceptualization by E.Y. and, data analysis by E.Y and S.S., investigations by G.F, S.M and S.D., writing and original draft preparation by E.Y and S.S., reviewing and editing by E.Y, S.S., S.M. and G.F. All authors have read and approved the final version of the article.
Reference
1-P. Chadwick and S. R. Podiatrist, “Fungal infection of the diabetic foot: the often ignored complication,” The Diabetic foot journal, vol. 16, pp. 102–107, 2013.
2- W. A. Ali, R. H. Hussein, and W. T. Radef, “The effect of Soil properties on the Biological Diversity of Fungi in Soil University of Anbar.,” in Journal of Physics: Conference Series, vol. 2114, no. 1, p. 012068 , 2021.
3-A. D. Association, “Standards of medical care in diabetes—2010,” Diabetes Care, vol. 33, no. Supplement_1, pp. S11–S61, 2010.
4-Pickwell K, Siersma V, Kars M, Apelqvist J, Bakker K, Edmonds M, Holstein, P, Jirkovská A, Jude E, Mauricio D and Piaggesi A. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care, 38(5): 852-857 , 2015 .
5-Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ and Schaper NC. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence based global consensus. Diabetes Metab Res Rev, 32(1): 2-6, 2016.
6-H. Kareliya, L. Bichile, A. Bal, A. Varaiya, and P. Bhalekar, “Fungal infection in diabetic foot a clinicomicrobiological study,” Acta Sci. Mcrobiology, vol. 2, pp. 49–55, 2019.
7- L. Kalan and E. A. Grice, “Fungi in the wound microbiome,” Adv Wound Care (New Rochelle), vol. 7, no. 7, pp. 247– 255, 2018.
8-Kwon-Chung, K.J. and Bennett, J.E. ,Cryptococcosis. In: Kwon-Chung, K.J. and Bennett, J.E., Eds., Medical Mycology, Lea & Febiger, Philadelphia, 397-446,1992.
9-Ellis D, Davis S, Alexiou H, Handke R and Bartley R , Description Of Medical Fungi. 2 nd ed. The National Library Of Australia Cataloguing –In Publication Entry,2007.
10-Soysa, N.; Samaranayake, L.; Ellepola, A, Diabetes mellitus as a contributory factor in oral candidiasis. Diabet Med. 23: 455-9.2006.
11-Akpan, A.and Morgan, R. Oral Candidiasis.Postgrad Med J.78:455-9, 2002.
12-Horn, D.L.; Neofytos, D.; Anaissie, E.J, Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis . 48: 1695–703, 2009.
13-Davies ,A.N.; Brailsford ,S.; Broadley ,K.and Beighton ,D. Oral yeast carriage in patients with advanced cancer. Oral Microbiol Immunol. 17:79,2002.
14-Belazi ,M.; Velegraki ,A.; Koussidou ,T.; Andrealis, D.; Hini ,S.; Arsenis ,G.; Eliopoulou ,C.; Destouni ,E. and Antoniades ,D. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol Immunol. 19: 347, 2004.
15-Saunte, D. M., Holgersen, J. B., Haedersdal, M., et al. Prevalence of Toe Nail Onychomycosis in Diabetic Patients. Acta. Derm. Venereol. 86: 425-428,2006.
16-H. Larone, Medically Important Fungi—A Guide to Identification, American Society for Microbiology, Washington, DC, USA, 3rd edition, 1995.
17-Hayat AS, Khan AH, Masood N. and Shaikh N. Study for microbiological pattern and in vitro antibioticsusceptibility in patients having diabetic foot infections at Tertiary Care Hospital in Abbottabad. World Appl. Sci. J.;12:123-131.6,2011.
18-Hena VJ and Growther L. Studies on bacterial infectionsof diabetic foot ulcer. Afr. J. Clin. Exp. Microbiol;11(3):146-149,2010.
19- S. A, Kannan N, Rajan K, Pramodhini M, RamanathanM. clinical study on the prevalence of fungal infections indiabetic foot ulcers. IJCRR.;7(23):08-13, 2015.
20-Tligui H, Oudaina W, El Ftouh S, et al. A skin ulcer infection due to Rhodotorula mucilaginosa in an immunocompromised child. J Mycol Med.28:215–217,2018.
21-Poradzka A, Jasik M, Karnafe W and Fiedor P.Clinical Aspects of Fungal Infections in Diabetes. Acta Poloniae Pharmaceutica n Drug Research 70(4), 587-596,2013.
22-Bhavan, P.S.; Rajkumar, R.; Radhakrishnan, S.; Seenivasan, C. and Kannan, S. Culture and identification of Candida spp. from vaginal ulcer and separation of enolase on SDS-PAGE. Inter. J. Bio. 2; (1): 84-93,2010.
23- H. Miceli, J. A. D´ıaz, and S. A. Lee, “Emerging opportunistic yeast infections,” The Lancet Infectious Diseases, vol. 11, no. 2, pp. 142–151, 2011.
24-Koneman, E.W. and Roberts, G.D. Micologia: Practica de Laborató- rio.3ed. Editorial Medica Panamericana, Buenos Aires. 221p,1990.
25-Larone, D. H. Medically important fungi. A guide to identification, 3rd ed.ASM Press, Washington, D.C,1995.
26-Larone, D.H., Medically Important Fungi—A Guide to Identification, 4th edn, ASM Press, Washington, DC, 2002.
27-Larone DH. Medically important fungi. 4th ed. Washington, D.C.: ASM Press; 2002.
28-P. G. Pappas et al., “Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America,” Clinical Infectious Diseases, vol. 62, no. 4. Oxford University Press, pp. e1–e50, Nov. 04, 2015.
29-D. Dicker et al., “Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: A systematic analysis for the Global Burden of Disease Study 2017,” The Lancet, vol. 392, no. 10159, pp. 1684–1735, Nov. 2018.
